BASIC STRUCTURE OF AN ATOM FOR KIDS
This post explains the basic structure of an atom, focusing on the subatomic particles (electrons, protons, and neutrons) present in it.
INTRODUCTION TO THE BASIC STRUCTURE OF AN ATOM
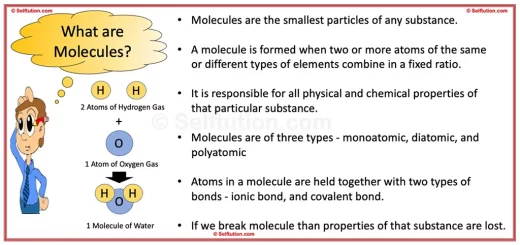
Atoms are the basic building blocks of matter. They are the smallest particles that have all the features and properties of a particular element.
Although atoms are incredibly small, they can be broken down into smaller parts: electrons, protons, and neutrons. However, when an atom is broken apart, it loses the characteristics of the element it belongs to.
Before we explore the basic structure of an atom, let’s learn about the discovery of these subatomic particles.
TOPICS COVERED:
- Discovery of subatomic particles
- The model of an atom
- Properties of electrons, protons, and neutrons
- Atoms of different elements
- The structural stability of an atom
- Electron Shells
- Fundamental or elementary particles

Representation of an Atom
DISCOVERY OF SUBATOMIC PARTICLES
For a long period, scientists believed that atoms could not be broken down into smaller particles. However, this changed after the discovery made by J.J. Thomson in 1897. In his quest to understand the basic structure of an atom, he discovered that atoms are divisible and contain a negatively charged subatomic particle called the electron.’ To know more about J.J. Thomson’s discovery, click here.
Soon after J.J. Thomson’s discovery, other scientists began racing to uncover the basic structure of an atom.
- In 1898, Goldstein concluded that since all atoms are electrically neutral, there must be positively charged particles present in them. To learn more about Goldstein’s experiment, click here.
- Rutherford discovered the nucleus in 1911 and protons in 1917. He further stated that these positively charged particles join together to form a ‘nucleus,’ which is present at the center of an atom with all electrons revolving around it. To know more, click here.
- In 1932, James Chadwick studied the atomic model and discovered particles without charge but a mass of almost protons. He named them ‘neutrons.’ To know more, click here.
Back to the introduction – The structure of an atom
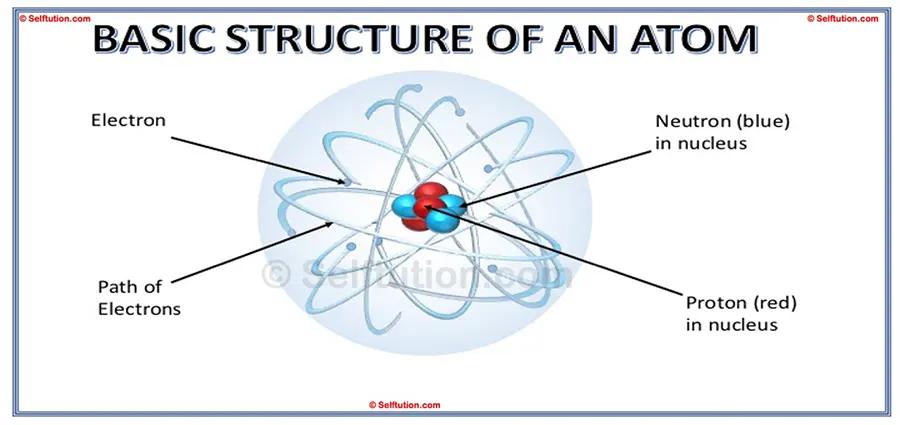
THE BASIC STRUCTURE OF AN ATOM
According to the modern standard model, the basic structure of an atom is as follows:
- An atom consists of three subatomic particles: electrons, protons, and neutrons.
- The two main parts of an atom are the nucleus and the electron shells, or orbits.
- The nucleus is the central part of the atom and contains protons and neutrons. Protons are positively charged particles, similar to tiny batteries with only a positive charge. Neutrons are the same size as protons but have no electrical charge. Since protons have a positive charge, they repel each other, but neutrons help to keep them together. The entire mass of an atom is mostly in the nucleus because electrons have very little mass compared to protons and neutrons. A proton and a neutron each have a mass approximately 1837 times greater than that of an electron.
- Electrons, much smaller than protons and neutrons, do not reside in the nucleus. Instead, they move around the nucleus in imaginary paths called orbits or shells. Each orbit has a fixed amount of energy, so these orbits are also called energy levels. Electrons in outer shells have more energy than those in inner shells. Each electron carries a negative charge, which balances the positive charge of the protons.
- Typically, an atom has an equal number of protons and electrons, making the atom electrically neutral since the positive and negative charges cancel each other out.
PROPERTIES OF ELECTRONS, PROTONS, AND NEUTRONS
ELECTRONS
- Electrons are an integral part of all atoms.
- An Electron has a definite mass, and it carries a definite electric charge.
- The mass of an electron is 1/1837 of the mass of a hydrogen atom (9.108 x 10-28 g).
- It has one unit negative charge equal to 1.602 x 10-19 Coulombs.
- An electron is denoted by the symbol -1e0. The superscript ‘0’ represents its mass, and the subscript ‘-1’ represents one unit of negative electrical charge.
PROTONS
- The mass of a proton is nearly equal to the mass of an atom of hydrogen, i.e., 1.672 x 10-24 g.
- The positive charge with a proton is equal to the negative charge on an electron, i.e., 1.602 x 10-19 Coulombs.
- A proton is denoted as +1p1, where the superscript ‘1’ represents 1 amu (atomic mass unit) mass and the subscript ‘+1’ represents one unit of positive charge.
NEUTRONS
- The mass of a neutron is slightly more than that of a proton, i.e., 1.676 x 10-24 g compared to 1.672 x 10-24 g.
- Electrically, a neutron is neutral, which means it has no charge.
Back to the introduction – The structure of an atom
ATOMS OF DIFFERENT ELEMENTS
There are 118 different types of atoms, and these atoms form the 118 basic substances known as elements. In every atom of each chemical element, the subatomic particles are identical. This means that the electrons in an iron atom are the same as in an oxygen atom. Similarly, the protons in carbon atoms are the same as the protons in hydrogen atoms, and the neutrons in an atom of carbon are the same as those in iron. What makes each chemical element unique is the number of subatomic particles it contains.
The basic structure of an atom differs for each element based on the number of protons, neutrons, and electrons it has. For instance, the smallest atom, hydrogen, contains only one proton and one electron, with no neutrons. On the other hand, the largest known atom, Oganesson (also known as Ununoctium), has 118 protons, 118 electrons, and approximately 176 neutrons. This difference in the number of subatomic particles gives each element its distinct properties.
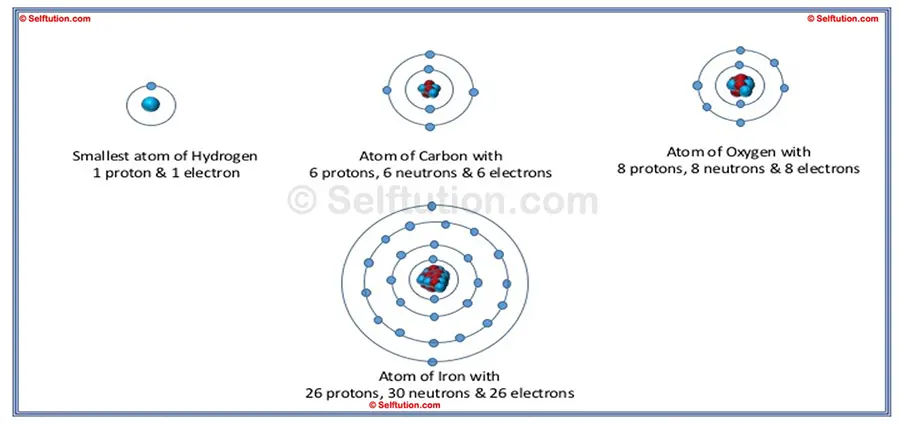
The Basic Structure of Hydrogen, Carbon, Oxygen, and Iron Atoms
STRUCTURE STABILITY OF AN ATOM
We know there is a force of attraction between particles with opposite electrical charges. Therefore, the attractive force between the electrons and protons within an atom exists. Electrons revolve around the nucleus at a fixed distance. To avoid falling into the nucleus, electrons move at a very high speed—approximately 3/4 of the speed of light. Electrons with lower energy levels orbit closer to the nucleus, while those with higher energy levels orbit farther away.
However, one might expect that electrons, being light, charged, and constantly in motion, would gradually lose energy, move closer to the nucleus, and eventually fall into it, causing the atom to collapse. But this does not happen.
According to Bohr’s theory, electrons revolve in fixed orbits or shells around the nucleus at very high speeds, with each orbit associated with a fixed amount of energy. Electrons in these shells neither lose nor gain energy unless an external force is applied. This stability maintains their position. As a result, the moving electrons counterbalance the inward force exerted by the nucleus with their outward force, preventing them from falling into the nucleus and ensuring the structural stability of the atom.
Back to the introduction – The structure of an atom
ELECTRON SHELLS
To understand the basic structure of an atom, it is important to have a good knowledge of electron shells. Charles Barkla first introduced the concept of an electron shell. He identified seven electron shells and labeled them with the letters K, L, M, N, O, P, and Q. These shells are numbered 1 through 7, from the innermost to the outermost shell. The closest shell to the nucleus is the ‘K-shell’ or ‘1-shell’, followed by the ‘L-shell’ or ‘2-shell’, and so on. The farthest shell from the nucleus is the ‘Q-shell’ or ‘7-shell’.
Each shell can hold only a fixed number of electrons, determined by the formula 2n², where ‘n’ stands for the numeric label of the shell. According to this formula, the maximum number of electrons in the K-shell is 2, in the L-shell is 8, in the M-shell is 18, in the N-shell is 32, in the O-shell is 50, in the P-shell is 72, and in the Q-shell is 98.
Although the formula provides the theoretical maximum, in practice, this maximum is only reached for the first four shells (K, L, M, N). No known element has more than 32 electrons in any one shell due to the presence of subshells.
To learn more about electron shells, click here
FUNDAMENTAL OR ELEMENTARY PARTICLES
Atoms were once believed to be the fundamental or elementary particles of the universe. However, this belief changed after scientists unraveled the basic structure of an atom and discovered subatomic particles such as electrons, protons, and neutrons. Advances in science have revealed that besides electrons, protons, and neutrons, there are many other subatomic particles, including muons, gluons, gravitons, and dozens of others. Furthermore, protons and neutrons are not the smallest particles; they consist of even tinier pieces called quarks. There are six kinds of quarks with odd names like up, down, strange, charm, bottom, and top. Quarks, with a group of particles called leptons, which include electrons, are probably the smallest pieces of matter. Scientists consider these particles to be fundamental or elementary.
What we learned above was very basic about the structure of an atom. Keep following for related posts.