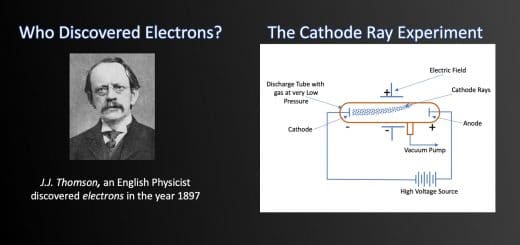
Who Discovered Electrons? – The Cathode Ray Experiment
J.J. Thomson, an English discovered electrons in the year 1897.

J. J. Thomson, the one who discovered electrons
For a long period in history, scientists were of opinion that atoms could not be broken further. But things changed after the discovery made by an English scientist named J.J. Thomson in the year 1897. In his quest to study properties of cathode rays, he discovered that atoms contain negatively charged subatomic particles – ‘electrons’. Thus, they are divisible.
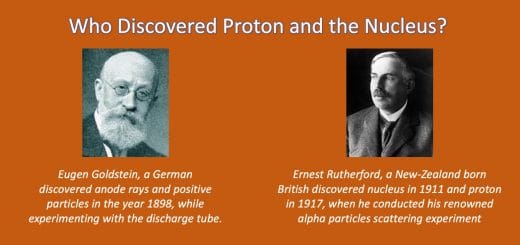
Soon, after J.J. Thomson discovered electrons, the race began among other scientists to uncover the basic structure of an atom. This led to the discovery of the nucleus and other subatomic particles – protons and neutrons.
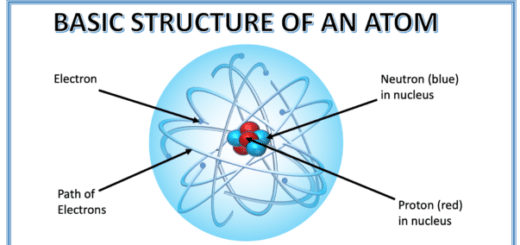
To know about the basic structure of an atom, click here
Who discovered electrons
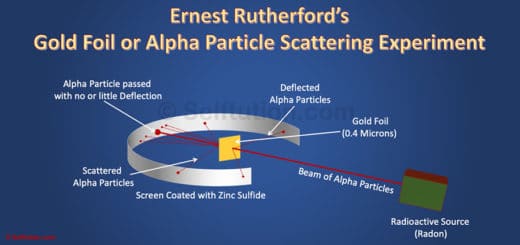
CATHODE RAY EXPERIMENT
Scientists in the early nineteenth century were aware of electricity and the effect of electric potential. The electric potential is a driving force that results in the flow of current through a substance due to the difference in concentration of charges at two ends of it. The magnitude of the electric current flowing through a substance is directly proportional to the electric potential applied across it. They proved that the electricity or the electric current can flow through any substance – solid, liquid or gas if enough driving force or electric potential exists. After carrying out a series of experiments with – solids, liquids, and gases, scientists took one step further to drive electricity through a vacuum. However, most of them failed as none of them were able to create a perfect vacuum.
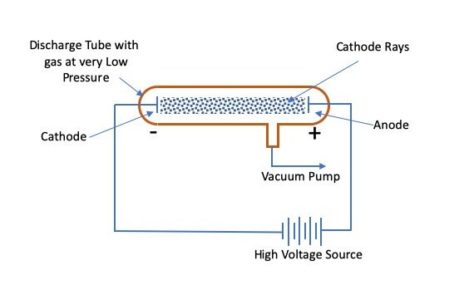
William’s Cathode Ray Experimental Setup

William Crook is the first person to pass an electric current through a vacuum
William Crooke, the British Scientist, in the year 1875 successfully created a near to perfect vacuum (0.01 mm of mercury) in a glass tube sealed at both ends with metal plates. He named it the discharge tube. William observed that when he applies a high electric potential across the discharge tube, then current flows from the negative terminal to the positive terminal in the form of rays. He called these ‘cathode rays’. Although, he could not establish what electric current or cathode rays comprise and what is moving from the negative terminal to the positive terminal.
Finally, in 1897 J. J Thomson discovered electrons while studying characteristics of cathode rays. He discovered that cathode rays consist of negatively charged subatomic particles (now called electrons), present in all atoms of the elements.
DISCOVERY OF ELECTRONS – THOMSON EXPERIMENT AND RESULT
In his experiment, J. J. Thomson applied an electric field in the path of cathode rays in the discharge tube. He observed that cathode rays were deflected towards the positive plate of the electric field. This showed that cathode rays consist of negatively charged particles.
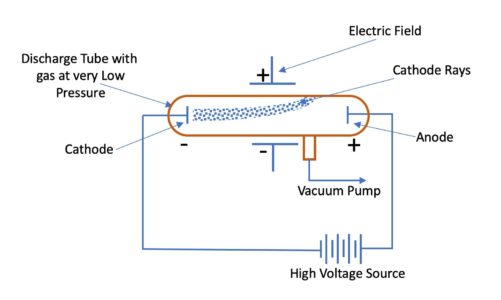
Deflection of cathode rays toward positive plate proves that they consist of negatively charged particles
Next, he applied a magnetic field in the path of cathode rays. He noted that cathode rays again deflects in a direction in which moving negative charge would be deflected. This again proved that cathode rays contained negatively charged particles called electrons.
The below video beautifully demonstrate the effect of a magnetic field on cathode rays. Courtesy kosasihiskandarsjah
https://www.youtube.com/watch?v=XU8nMKkzbT8
Johnson further noted that the amount of deviation in cathode rays is directly proportional to the strength of the magnetic or electric field applied. Using this principle he measured the mass to the charge ratio (m/q) of the particles present in the cathode rays. He also noted that this ratio of mass to the charge (m/q) is constant and does not get affected by the type of gas in the discharge tube. This again proved that cathode rays consist of particles of the same type.

Robert Andrews Millikan is the one who discovered the value of charge on electrons
Although Johnson discovered electrons he failed in measuring either its mass (m) or the charge (q) separately.
The exact mass (m) of the electrons was discovered later on in the year 1911, when another scientist Robert Andrews Millikan, an American successfully calculated the minimum charge (q) which can be carried by any particle.
SYMBOL AND PROPERTIES OF AN ELECTRON
The symbol used to denote electron is -1e0. The superscript ‘0’ represents its mass and the subscript ‘-1’ represents it’s one unit negative electrical charge.
- Electrons are an integral part of all atoms.
- An Electron has a definite mass and it carries a definite electric charge.
- The mass of an electron is 1/1837 of the mass of a hydrogen atom (9.108 x 10-28 g).
- It bears one unit negative charge which is equal to 1.602 x 10-19 Coulombs.
THOMSON MODEL OF AN ATOM – PLUM PUDDING MODEL
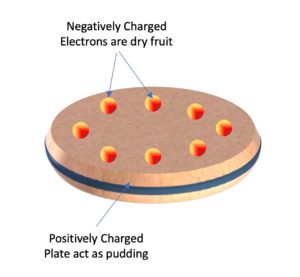
Thomson’s Plum-Pudding Model
After J.J. Thomson discovered negatively charged electrons in 1897, Eugen Goldstein, a German concluded that since all atoms are electrically neutral, thus there must be positively charged particles present in them. He discovered positively charged particles in the year 1898 while experimenting with a discharge tube, although with slight variation.
In 1904, J. J. Thomson worked out the first model of an atom – the Plum Pudding Model.
According to this model, an atom is a positively charged sphere in which electrons are embedded just like dry fruits are distributed in a pudding. Therefore it is known as the Plum Pudding Model.
Since the total positive charged of the atom was equal to the total negative charge of its electrons, it followed that an atom would become negatively charged if it gained electrons and positively charged if it lost electrons. However, this model failed to explain many experimental observations about atoms.









2 Responses
[…] a high electric potential across the discharge tube he noted the presence of another ray along with cathode rays. These rays were traveling as waves in the opposite direction i.e. from anode to the cathode. Some […]
[…] For a long period in history, scientists were of opinion that atoms could not be broken further into smaller particles. But things changed after the discovery made by a scientist named J.J. Thomson in the year 1897. In his quest to ascertain the basic structure of an atom, he discovered that atoms are divisible and contain a negatively charged subatomic particle – ‘electron’. To know more about J.J. Thomson’s discovery, click here. […]