Elements and Compounds | Simplified for kids
Kids often ask, what are elements and compounds? So here is the basic definition for an element and a compound which kids can easily remember.
Element is a substance, which have only one kind of atoms, whereas compound has two or more different kinds of atoms.
Topics Covered:
- Introduction
- What is an element?
- What is a compound?
- Properties of element and compound
- Formulae of elements and compounds
- Difference between an element and a compound
INTRODUCTION
There are millions of substances in this world. In chemistry, we study the nature and property of these substances, so we can use them for the benefit of mankind. However, it is not possible to study all of them one by one. Thus, we divide them into – elements and compounds.
Skip to >> The difference between element and compound for kids
WHAT IS AN ELEMENT?
Definition of an element for kids –
An element is a substance that cannot be broken into two or more simpler substances by any physical or chemical method. It consists of atoms of the same kind.
The elements are the basic substances, which make all kinds of different substances around us. We call elements a basic substance because we cannot divide them into two or simpler substances by any physical or chemical method. An element consists of only one kind of atom. Therefore, how much we break it; we will end up with the same kind of atoms.
Let’s understand with an example-
 To build any house, we need mud, cement, bricks, sand, steel, and wood. By using these materials in different quantities and ratios, we can either make a simple house or a huge building. Much in the same way, in nature, there are elements, which combine to make all kinds of substances.
To build any house, we need mud, cement, bricks, sand, steel, and wood. By using these materials in different quantities and ratios, we can either make a simple house or a huge building. Much in the same way, in nature, there are elements, which combine to make all kinds of substances.
Hydrogen, helium, carbon, oxygen, calcium, nitrogen, sodium, iron, silver, gold, and mercury are a few examples of elements. So far we have discovered total – 118 types of elements. 92 elements out of 118 occur naturally in rocks, soil, air, and water. While scientists create the remaining 26 artificially in a lab.
Symbols of Elements
Scientists use symbols to represent various elements, instead of their names. For example, (H) stands for Hydrogen, (O) for Oxygen, (C) for Carbon, (N) for nitrogen, and so on. We use symbols because when different elements combine to form compounds then it is easy to represent them by symbols.
Let’s understand it by an example,
To represent a molecule of sugar we can write either as ‘C12H22O11‘ or as ‘12 atoms of carbon, 22 atoms of hydrogen, and 11 atoms of oxygen’. Clearly, the former method is better.
For a complete list of elements and their symbols, refer to the periodic table. The periodic table gives the complete list of 118 elements along with other important information.
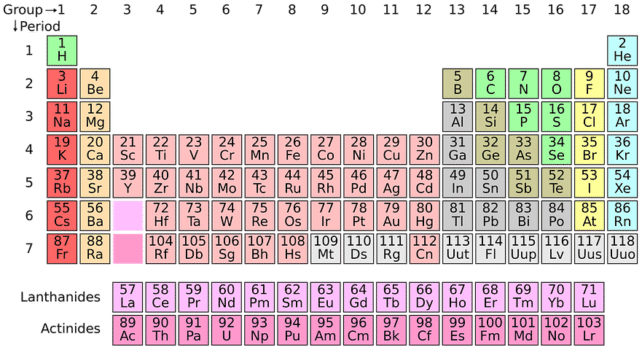
Periodic Table
Molecules of elements
A molecule of an element can be monoatomic, diatomic, or polyatomic, but it will always have atoms of the same kind. For example – Helium (He), Hydrogen (H2), Phosphorous (P4), Sulphur (S8), etc.
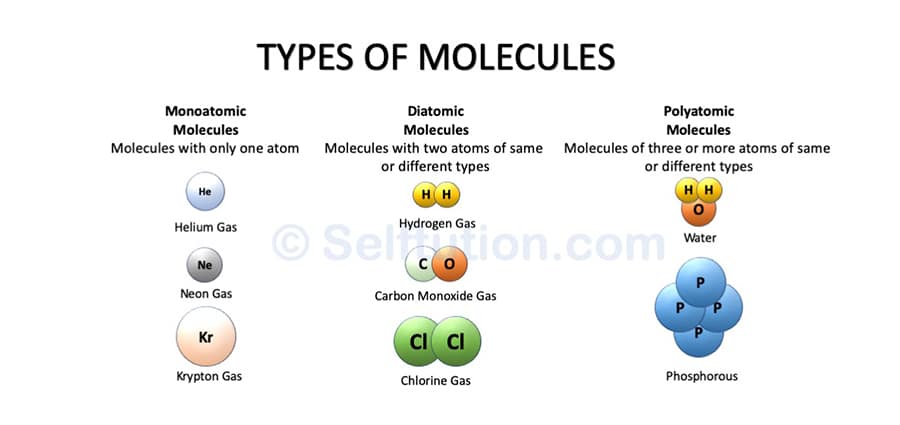
Examples of different types of molecules – Monoatomic, Diatomic, and Polyatomic
Skip to >> The difference between element and compound for kids
WHAT IS A COMPOUND?
Definition of a compound for kids-
When a molecule of a pure substance contains atoms of two or more elements combine in the fixed ratio, it is said to be a compound.
To understand compound first consider this fact. We know that the English alphabet has 26 letters. By combining these 26 letters, we can make millions of English words. Much the same way you can combine 118 different kinds of elements to make an endless number of compounds. Therefore, a compound is a substance formed when two or more elements combine in a fixed ratio. It is a new substance formed from its elements. For example, two atoms of the element hydrogen (H) and one atom of the element oxygen (O) combine to form a molecule of water (H2O), which is a compound.
We can break a molecule of a compound to get pure elements. However, this breakup is not possible with simple physical methods. In compounds, chemical bonds join atoms together. These bonds are very strong and difficult to break. Thus, to get original elements (or atoms) from compounds we need to apply chemical methods. For example – to break the molecule of water into its elements hydrogen and oxygen, we need to pass an electric current through it.
https://www.youtube.com/watch?v=qHxDHu9wGAw
Video courtesy: Alternate Learning
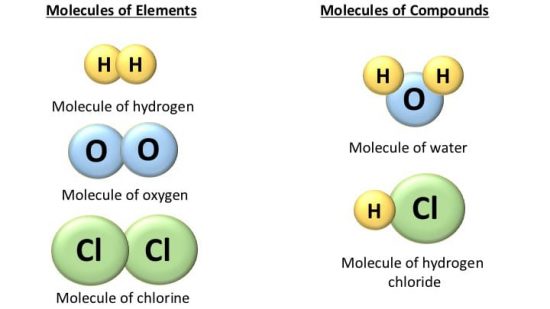
Molecules of Compounds
The molecule of the compound always contains two or more atoms of different kinds. For example,
- Hydrogen (H) and oxygen (O) make water (H2O),
- Carbon (C) and oxygen (O) make carbon dioxide (CO2),
- Hydrogen(H) and chlorine (Cl) make hydrochloric acid (HCl).
PROPERTIES OF ELEMENT AND COMPOUND
Compounds have properties, which are completely different from the properties of elements that make them. For example – a molecule of water (H2O) is a compound that has two atoms of hydrogen (H) and one atom of oxygen (O). Water is liquid under normal conditions while hydrogen and oxygen are gases. A mixture of hydrogen and oxygen when ignited it creates fire while another way round we use water for extinguishing a fire.
FORMULAE OF ELEMENTS AND COMPOUNDS
Formulas are a short way of representing molecules of elements and compounds. They help in understanding the structure of molecules. Formulas are more relevant for compounds than elements as they consist of atoms of different elements. The formula for compound gives the following information:
- It tells which elements are present in a compound, and
- It tells the number of atoms of each element present in a compound.
For example, the formula ‘H2O’ represents water. It tells us that two atoms of hydrogen and one atom of oxygen makes a molecule of water.
DIFFERENCE BETWEEN ELEMENT AND COMPOUND FOR KIDS
- Elements consist of only one kind of atoms, whereas compounds always have more than one kind of atoms.
- We cannot divide elements into two or simpler substances by physical or chemical means, whereas we can divide compounds into elements by chemical means.
- We use a symbol to represent an element, whereas chemical formula is used to represent a compound.
- There are only 118 types of elements known to mankind, whereas there is no upper limit on the type of compounds that can be formed.
Difference between element and compound in tabular form:
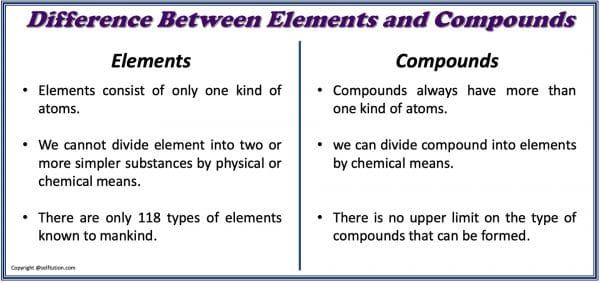
Difference Between Element and Compound













I’m gone to convey my little brother, that
he should also go to see this web site on regular basis to take updated from hottest information.
Its not my first time to go to see this web site, i am visiting this web page
dailly and obtain good information from here all the time.
Keep this going please, great job!
Thanks for sharing your thoughts on website.
Regards
My brother recommended I might like this web site.
He used to be entirely right. This put up actually made my day.
You cann’t believe just how a lot time I had spent for this information! Thank you!
Thanks
i had this for 6th grade science, and it is very fun. would reccomend,
8th grade science