Valency and Variable Valency | Valence Shell and Electrons
In chemistry, we frequently encounter terms like valency, variable valency, valence electrons, and valence shells. All of these terms explain combining the capacity of an atom of an element with the atoms of other elements to form a molecule.
Topics covered:
- Definition of valency, variable valency, valence shell & valence electrons
- Why do atoms combine to form molecules?
- How do atoms combine?
DEFINITIONS
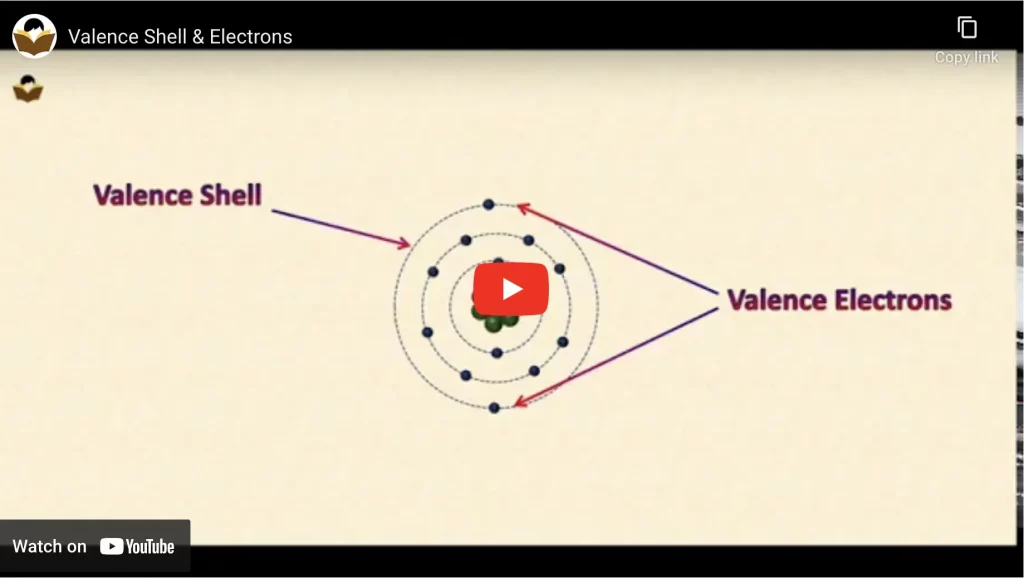
Definition of Valence Shell
The outermost shell of an atom is known as its valence shell or valance orbit.
Definition of Valence Electrons
Valence electrons are the one which is present in the outermost or valance shell of an atom.
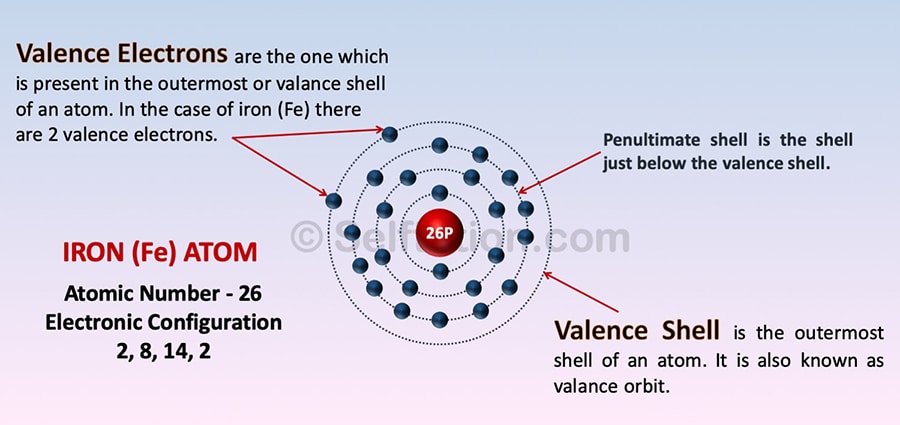
Valence Shell and Electrons of the Iron atom
Definitions of Valency
Valency is the combining capacity of an atom of an element or of ion or a radical with the atoms of other elements or radicals to form molecules.
Or
It is the number of electrons that an atom can lose, gain, or share during a chemical reaction.
Or
Valency of an element or radical is the number of hydrogen atoms, which can combine with or displace one atom of the element or radical, forming a compound.
Definition of Variable Valency
When certain elements exhibit more than one valency, this means they show variable valency.
Why do atoms combine to form molecules?
All atoms except those of inert gases are chemically unstable. By chemically unstable, we mean that they cannot exist freely in nature. Therefore, they try to stabilize by combining with other atoms of the same or different elements using the octet or duplet rule.
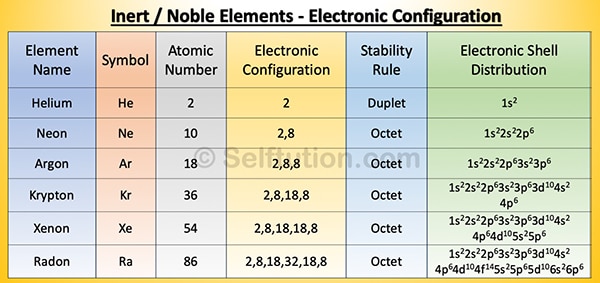
Inert Elements: Electronic Configuration and Distribution
As per the octet rule, to be chemically stable, an atom of an element must have eight electrons in its outermost orbit or shell. Atoms with electrons less than eight in their outermost or valence shell are chemically unstable. Such, atoms gain or lose electrons to attain a stable electronic configuration. The electrons present in the valence shell of an atom are called valence electrons. If the atom has only one shell, as in the case of hydrogen and helium, the valence shell can have two electrons. This is called the duplet rule.
Therefore, atoms combine to form molecules to attain chemical stability because they have an incomplete valence shell.
How do atoms combine?
Atoms combine with two types of bonds – ionic (electrovalent) bonds and covalent bonds.
IONIC BOND: Atoms combine using the ionic bond when one of the atoms participating in molecule formation releases electrons in the valence shell and hands them over to the other atoms. Due to this, the atom that loses electrons becomes positively charged and the other that gains electrons becomes negatively charged. These newly formed positively and negatively charged particles are called ions. These ions bearing opposite charges attract each other with electrostatic force and result in the formation of molecules. This electrostatic force between ions that keeps them together is called an ionic or electrovalent bond.
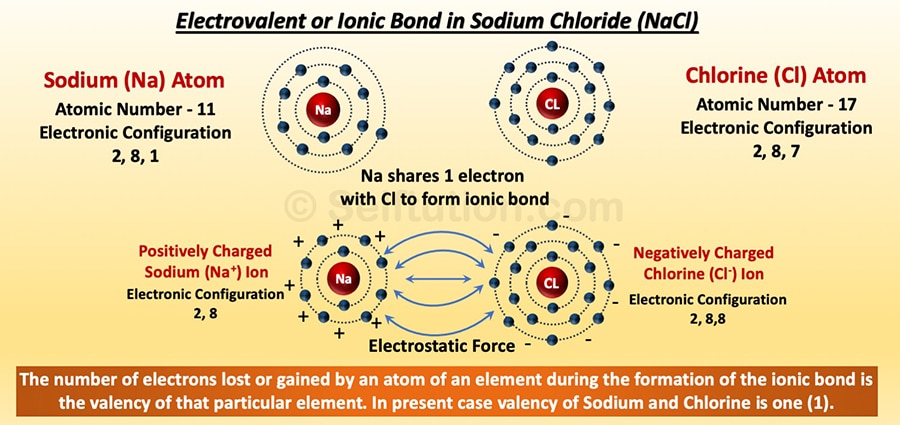
Electrovalent or Ionic Bond Formation in Sodium Chloride
COVALENT BOND: When atoms do not release or take electrons but share electrons among themselves, they result in the formation of a covalent bond. The number of electrons shared by an atom of an element during the formation of a covalent bond is the valency of that particular element.
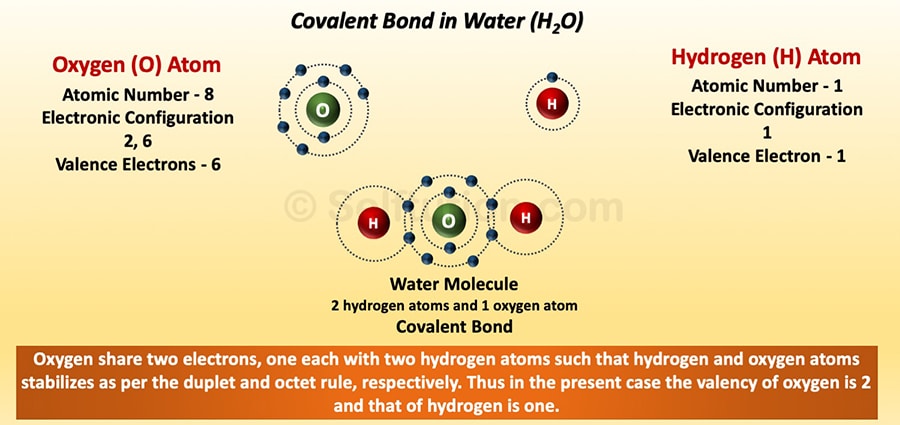
Covalent Bond in a Water Molecule
VALANCE SHELL & ELECTRONS
The outermost shell of an atom is known as its valence shell or valence orbit, and the electrons present in it are called valence electrons. The number of valence electrons varies from 1 to 8 for the atoms of the different elements.
The valence electrons of an atom determine the valency of that element. The knowledge of valence shells and electrons helps in understanding the formation of molecules of elements and compounds.
VALENCY
The valency of an element is a measure of its combining power with other elements.
Earlier, we used to measure valency in terms of hydrogen atoms. It is the number of hydrogen atoms that will combine with or displace one atom of a particular element or radical. As a hydrogen atom consists of only one valence electron in its outermost shell, it can readily lose or gain an electron to stabilize.
Some examples are,
- Valency of chlorine in hydrogen chloride: In the compound hydrogen chloride (HCL), one atom of chlorine combines with one atom of hydrogen; hence, the valency of chlorine is 1.
- Valency of sulfur in hydrogen sulfide: In the compound hydrogen sulfide (H2S), one atom of sulfur combines with two atoms of hydrogen; hence, the valency of sulfur is 2.
- Sulfate ion valency in sulfuric acid: In sulfuric acid (H2SO4), one ion of sulfate combines with two atoms of hydrogen; hence, the valency of sulfate is 2.
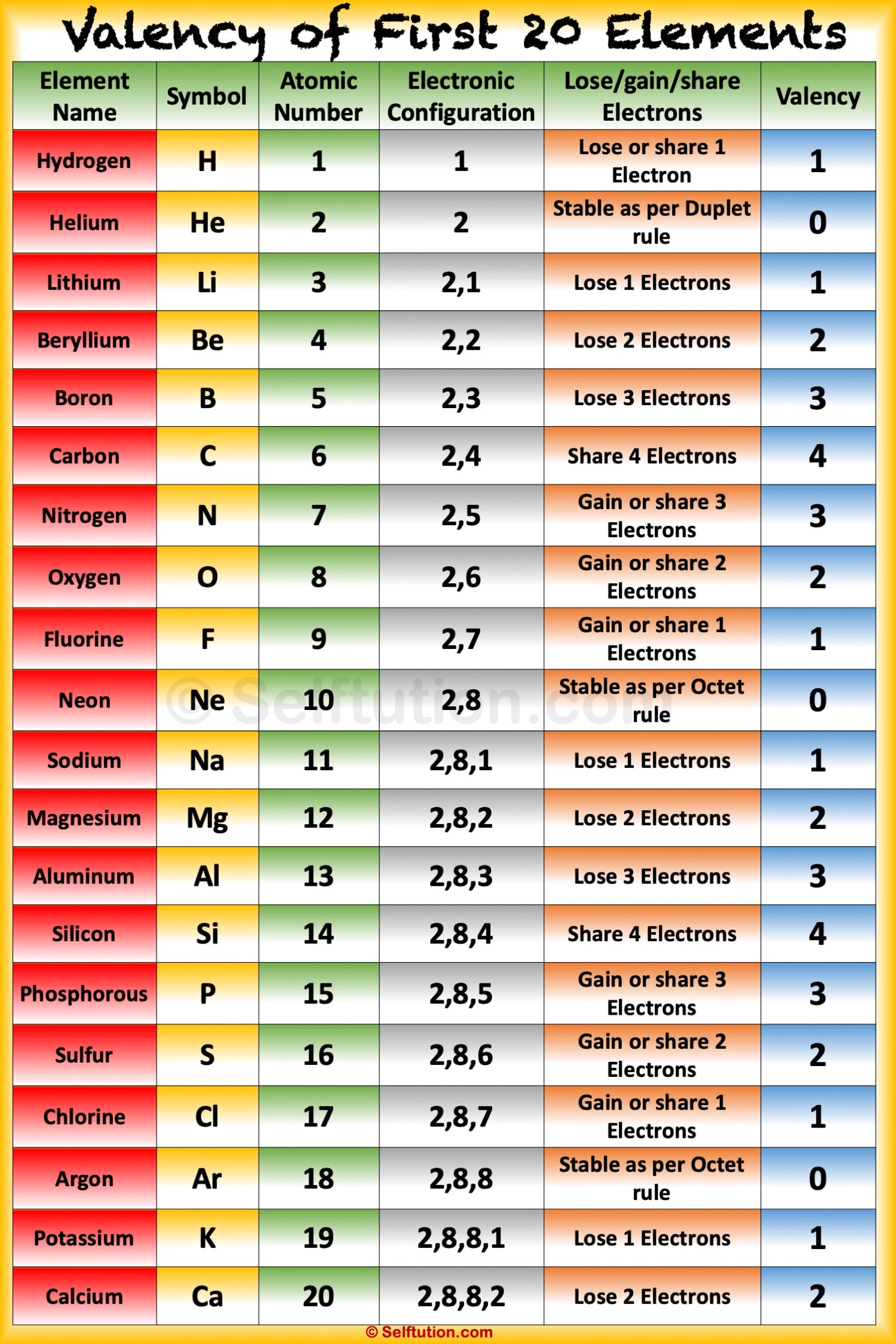
Valency of the First 20 Elements with Electronic Configuration
Modern Definition and Explanation of Valency
As per the modern definition, valency is the number of electrons that an atom can lose, gain, or share during a chemical reaction.
Explanation of valency–
As discussed above, atoms of all elements except those of inert gases are chemically unstable. To attain stability, atoms have to have eight (octet rule) or two (duplet rule) electrons in their valence shell. Atoms with electrons less than eight or two in their valence shell are chemically unstable. Such, atoms gain, lose, or share electrons to attain a stable electronic configuration. The loss, gain, or sharing of electrons by an atom depends upon electrons in the valence shell.
Thus, atoms with one, two, or three electrons in their valence shell prefer to lose or share these electrons to attain stability. Whereas, atoms with seven, six, or five electrons in their valence shell prefer to receive or share one, two, or three electrons, respectively.
It is important to note that we never depict valency with positive and negative symbols.
The atom or a group of atoms that lose electrons form positive ions. Whereas, those who receive electrons form negative ions. When atoms share electrons then they do not develop electric charges and remain neutral.
Monovalent: The atoms or a group of atoms with a valency of one are called monovalent. It means they gained, lost, or shared one electron to attain a stable electronic configuration of eight or two, as applicable.
Bivalent: The atoms or a group of atoms that gain, lose, or share two electrons are called bivalent.
Trivalent: These are atoms or a group of atoms with a valency of three. It means they gained, lost, or shared three electrons.
Tetravalent: The atoms or a group of atoms with a valency of four are called tetravalent. It means they gained, lost, or shared four electrons.
VARIABLE VALENCY
An atom of an element can sometimes lose more electrons than are present in its valence shell, i.e., there is a loss of electrons from the penultimate shell. Therefore, such an element is said to exhibit variable valency. Both metals and non-metals can exhibit variable valency. Some of the examples of variable valency are iron, copper, mercury, lead, tin, sulfur, phosphorus, etc.
Representation of variable valency of metals
In the case of metals, to exhibit variable valency, we represent the lower valency by adding the suffix ‘ous’ to the name of the metal; to represent the higher valency, we add the suffix ‘ic’. For example, the metal iron has valencies 2 and 3. For the lower valency (2), we write ferrous (Fe2+), and for the higher valency (3), we write ferric (Fe3+). Note that the symbol remains the same, but the name changes.
However, in the modern method, we represent the variable valency of an element by Roman numbers. Thus, to represent ferrous, we use Fe(II), and ferric, we use Fe(III).
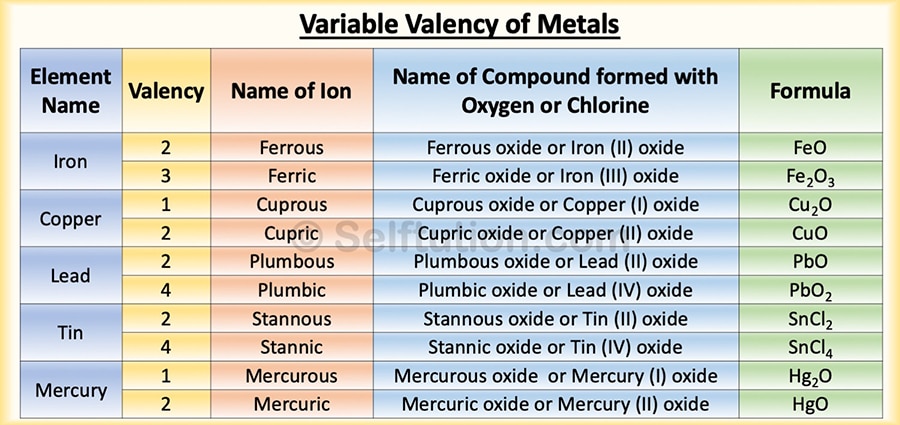
Variable Valency Examples for Metals
Representation of variable valency of nonmetals
In the case of non-metals, the number of other types of atoms attached to them determines their valency. For example, phosphorus has valencies 3 and 5. With chlorine, it forms two compounds, PCl3 and PCl5. Therefore, in the molecule of phosphorus trichloride, phosphorus shares three electrons with three chlorine atoms; thus, its valency is three. Whereas phosphorus pentachloride shares five electrons with five chlorine atoms, thus its valency is five.
For more such information, please visit are YouTube channel SELFTUTION