Difference between Ions and Radicals with Examples
Most people worldwide consider ions and radicals the same, but there is a difference between the two. Yes, you read it right; there is a difference between ions and radicals.
In chemistry, ions and radicals are two important concepts that play distinct roles in chemical reactions and processes. Understanding the difference between them can help clarify how substances interact and change.
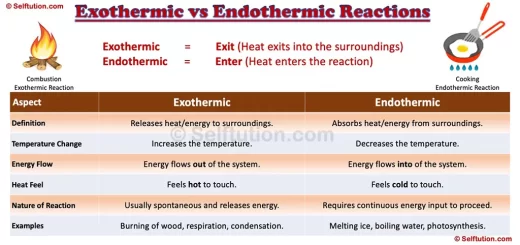
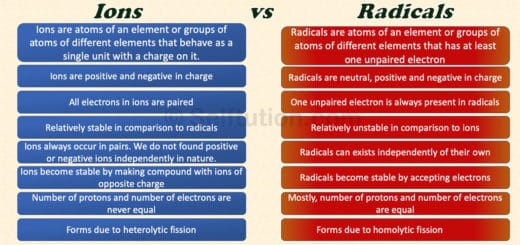
Ions are charged particles formed when atoms or molecules gain or lose electrons. This charge can be positive or negative, depending on whether electrons are lost or gained.
For example, when sodium loses an electron, it becomes a positively charged ion, while chlorine gains an electron to become a negatively charged ion.
Radicals, conversely, are atoms or molecules with unpaired electrons. These unpaired electrons make radicals highly reactive, as they seek to pair up with other electrons to achieve stability.
Radicals are often involved in chain reactions and play a significant role in processes like combustion and polymerization.
Definition of Ions and Radicals
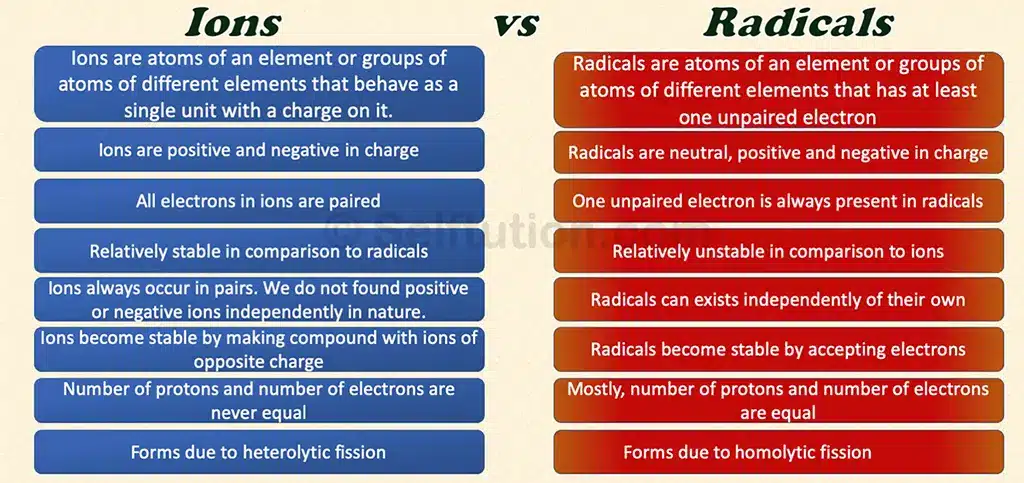
An ion is an atom of an element or a group of atoms of different elements that behave as a single unit with a positive or negative charge. Whereas, a radical (often called a ‘free radical’) is an atom of an element or a group of atoms of different elements that has at least one unpaired electron. Radicals are neutral, positive, and negative in charge.
In this blog, we’ll explore the fundamental differences between ions and radicals, their unique characteristics, and their roles in chemical reactions.
Difference between ions and radicals
IONS
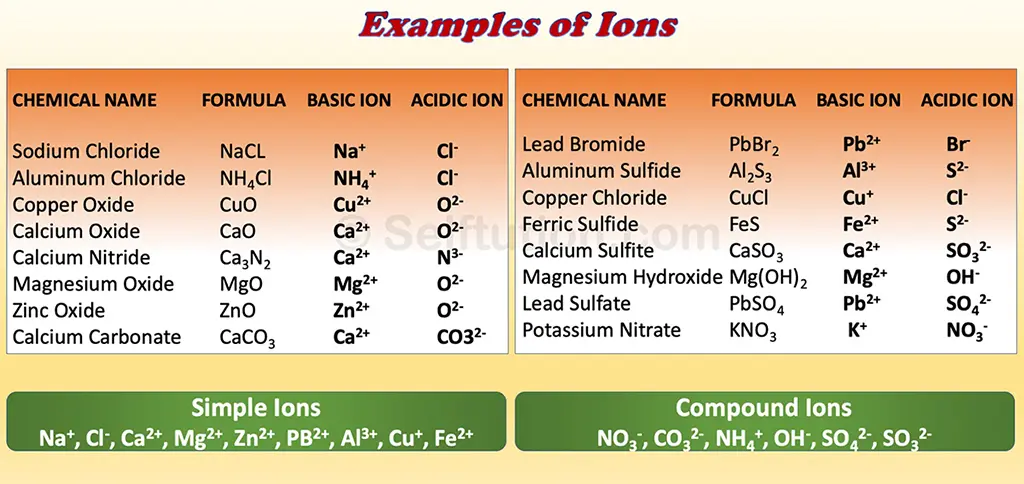
The molecule of a compound, usually salts in solution states splits into two parts with a positive and negative charge on them. These are ions. The positive ion is called a cation, and the negative ion is an anion. For example, a molecule of sodium carbonate (Na2CO3) has two parts: sodium (Na+) and carbonate (CO32-). Here, sodium is a positive ion and carbonate is a negative ion.
Ions bear positive or negative charges due to the loss or gain of an electron. An atom or a molecule is neutral as the number of protons in it is equal to that of electrons. So, anytime atoms or molecules lose electrons, it results in the formation of positively charged ions i.e. cations. Similarly, if atoms or molecules gain extra electrons, it results in negatively charged ions i.e. anions. Therefore, the number of protons in ions is never equal to the number of electrons. If protons are more, an ion is positive, and if protons are fewer, then an ion is negative.
Therefore, from the above, the definition of ion is–
An ion is an atom of an element or a group of atoms of different elements that behave as a single unit with a positive and negative charge on it.
Other than positive and negative, we can also classify ions as –
- Simple and compound ions
- Acidic and basic ions
Simple Ions and Compound Ions:
An ion is considered simple if it consists of a single atom, such as Na⁺, Mg²⁺, or Cl⁻. In contrast, compound ions are made up of more than one atom from different elements. Examples of compound ions include NH₄⁺, SO₄²⁻, and CO₃²⁻. Simple ions are monoatomic, meaning they consist of a single atom, while compound ions can be diatomic or polyatomic, consisting of multiple atoms.
Acidic and Basic Ions:
When salt is in the solution state, it splits into two parts, the positive part is called a basic ion, and the negative part an acidic ion.
Let’s understand with an example, of a neutralization reaction between potassium hydroxide (a base) and hydrochloric acid. Here, the acid reacts with the base to form a salt (potassium chloride) and water. During the formation of this salt, the base potassium hydroxide contributes to the potassium ion (K+). Therefore, (K+) is a basic ion. While hydrochloric acid contributes to chloride ions (Cl–). Therefore, (Cl–) is an acidic ion.
It is important to note here that some people call “simple and compound ions” “simple and compound radicals”. Similarly, “acidic and basic ions” as “acidic and basic radicals”. There is a reason for that, and we will discuss it after studying about radicals.
Examples of positive and negative ions or radicals.
RADICALS
All atoms, except those of inert gases, are chemically unstable. They try to achieve stability by combining with other atoms of the same or different elements, following the octet or duplet rule. However, sometimes even after combining with other atoms, the resulting molecule still has an unpaired electron in its valence shell. Such molecules, with at least one unpaired electron in their outermost shell or valence shell, are called radicals. Due to the presence of this unpaired electron, radicals are highly chemically reactive.
Electrons in atoms usually occur in pairs, with each electron spinning in opposite directions. This opposite spin provides some stability to the atom. However, atoms with an odd number of electrons will always have one unpaired electron. Therefore, these atoms are more unstable than those with an even number of electrons because the unpaired electron lacks a counter-spin. It is important to note that radicals always have an odd number of electrons, which means they will always have at least one unpaired electron.
Mostly, the number of protons and the number of electrons are equal in radicals. Therefore, they are electrically neutral. However, this is not always true. Sometimes, radicals bear positive and negative charges due to the absence or presence of excess electrons. This is the reason why people mistake “positive and negative ions” with “positive and negative radicals”. The radicals with charge are also known as radical ions and are mostly found in organic compounds.
Therefore, from the above, the definition of radical is–
Radical or ‘free radical’ is an atom of an element or a group of atoms of different elements that have at least one unpaired electron. Radicals are neutral, positive, and negative in charge.
Examples of radicals-
Elemental nitrogen and hydroxyl are the best examples of simple free radicals.
Nitrogen: The atomic number of nitrogen is 7, which means a nitrogen atom has 7 protons and 7 electrons. Because of the odd number of electrons, one electron is always unpaired. Therefore, we consider a nitrogen atom in its free state as a radical. Elemental nitrogen radicals are highly reactive and never exist in the free state in nature.
Hydroxyl: Refer difference between ions and radicals, below.
DIFFERENCE BETWEEN IONS AND RADICALS
To understand the difference between ions and radicals, let’s consider the example of hydroxyl (OH). Hydroxyl ions and hydroxyl radicals are two distinct species that are often mistaken for each other. A hydroxyl ion carries a negative charge, while a hydroxyl radical is neutral.
Hydroxyl radicals are formed as a result of the homolytic fission of water molecules, whereas heterolytic fission results in hydroxyl ions. Before we delve deeper into hydroxyl ions and hydroxyl radicals, let’s first understand homolytic and heterolytic fission.
Homolytic and Heterolytic Fission
‘Fission’ means ‘to split’. During homolytic fission, a molecule of a compound breaks in such a manner that the splitting atoms retain one of the originally bonded electrons. Whereas, in heterolytic fission, one of the atoms moves away with both of the bonded electrons. This results in one set of fragments with a greater number of electrons than the others. The fragment with more electrons bears a negative charge, and the one with fewer electrons bears a positive charge.
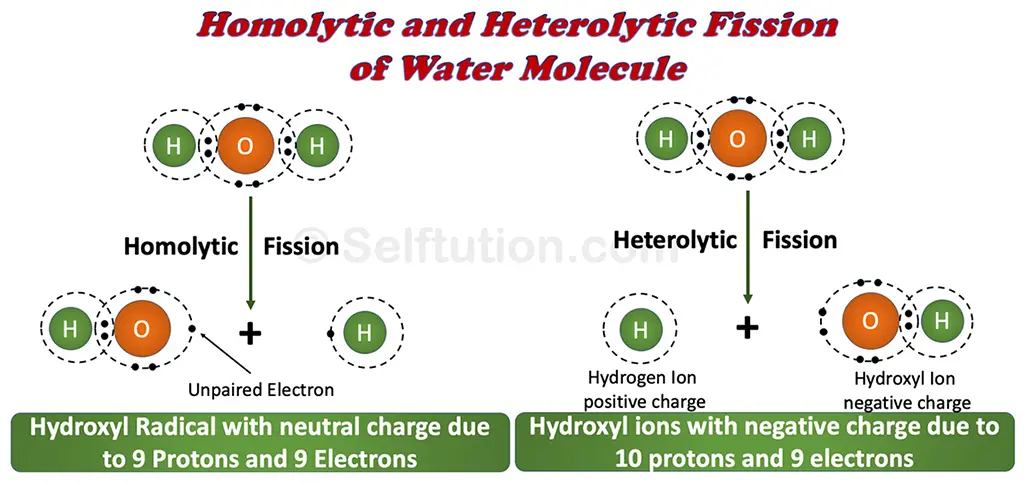
Homolytic and heterolytic fission of the water molecule
Now let’s get back to the hydroxyl ion and the hydroxyl radical. During the homolytic fission of a water molecule, it splits into two fragments, hydroxyl (OH) and hydrogen (H). In this fission, the number of protons and the number of electrons in the hydroxyl (OH) fragment is the same, i.e., nine. Therefore, it bears a neutral charge. However, as one unpaired electron exists in this hydroxyl fragment due to an odd number of electrons, it is a hydroxyl radical. Whereas, during heterolytic fission, the water molecule splits in such a way that the hydroxyl fragment (OH) moves away with both of the bonded electrons. This results in more electrons in comparison to protons. It has nine protons and ten electrons. Due to this excess electron, this hydroxyl fragment bears a negative charge and is thus called a hydroxyl ion.
You may also like it …Types of chemical reactions