Bohr’s Atomic Model for Hydrogen
Master Bohr’s Atomic Model for Hydrogen – Simplified Explanation with Diagrams & Formulas
Selftution.com – the #1 trusted educational website – breaks down Bohr’s model into easy steps, helping you grasp electron orbits, energy levels, and quantum jumps effortlessly.
Welcome to your ultimate learning hub, where complex science becomes simple!
Bohr’s atomic model is an essential concept in atomic physics. It helps explain how electrons move around the nucleus in specific orbits without collapsing into it.
Atoms are the fundamental units of matter. Understanding their structure helps us learn about chemical reactions and the behavior of different elements.
Before Bohr, earlier models of the atom had limitations. They could not fully explain the stability of electrons or the emission of specific light wavelengths.
In 1913, Niels Bohr proposed a model that addressed these issues. His theory introduced fixed orbits and quantized energy levels for electrons.
According to Bohr, electrons orbit the nucleus at specific distances. They cannot exist between these levels, making the atomic structure more predictable.
Bohr’s model was a breakthrough in atomic theory. It helped explain hydrogen’s spectral lines and set the foundation for modern quantum mechanics. In this article, we will explore Bohr’s atomic model in simple terms.
The Need for a New Atomic Model

Niels Bohr, a Danish physicist
Before Bohr introduced his model, scientists had different theories about atomic structure. The most famous ones before Bohr were J.J. Thomson’s Plum Pudding Model and Rutherford’s Nuclear Model. While Rutherford’s model suggested that atoms have a dense, positively charged nucleus with electrons moving around it, it did not explain why electrons did not collapse into the nucleus due to attraction.
Bohr’s atomic model addressed this problem and provided a clearer explanation of how electrons are arranged in an atom, particularly in the hydrogen atom.
Bohr’s Postulates (Main Ideas)
Bohr proposed his model for the hydrogen atom, consisting of only one electron and one proton. His theory was based on the following important postulates:
1. Electrons Move in Fixed Orbits
Bohr suggested that electrons do not move randomly around the nucleus. Instead, they revolve in fixed circular paths called orbits or energy levels. These orbits are like the planets revolving around the Sun, but they are governed by quantum physics.
2. Quantization of Energy Levels
Each orbit has a fixed amount of energy, which means that electrons can only exist in these specific orbits and not in between them. The energy levels are labeled as K, L, M, N… or numbered as n = 1, 2, 3, etc.
3. Energy Absorption and Emission
An electron can move from a lower energy level to a higher one by absorbing energy. Similarly, it can return to a lower level by releasing energy through light (photons). This explains why hydrogen and other elements produce specific colors when heated or energized.
4. Angular Momentum is Quantized
Bohr explained that the angular momentum of an electron is fixed and follows the formula:
mvr = nh / 2π
where:
- m = mass of the electron
- v = velocity of the electron
- r = radius of the orbit
- h = Planck’s constant
- n = energy level (1, 2, 3…)
This means that electrons can only exist in certain permitted orbits and not in between.
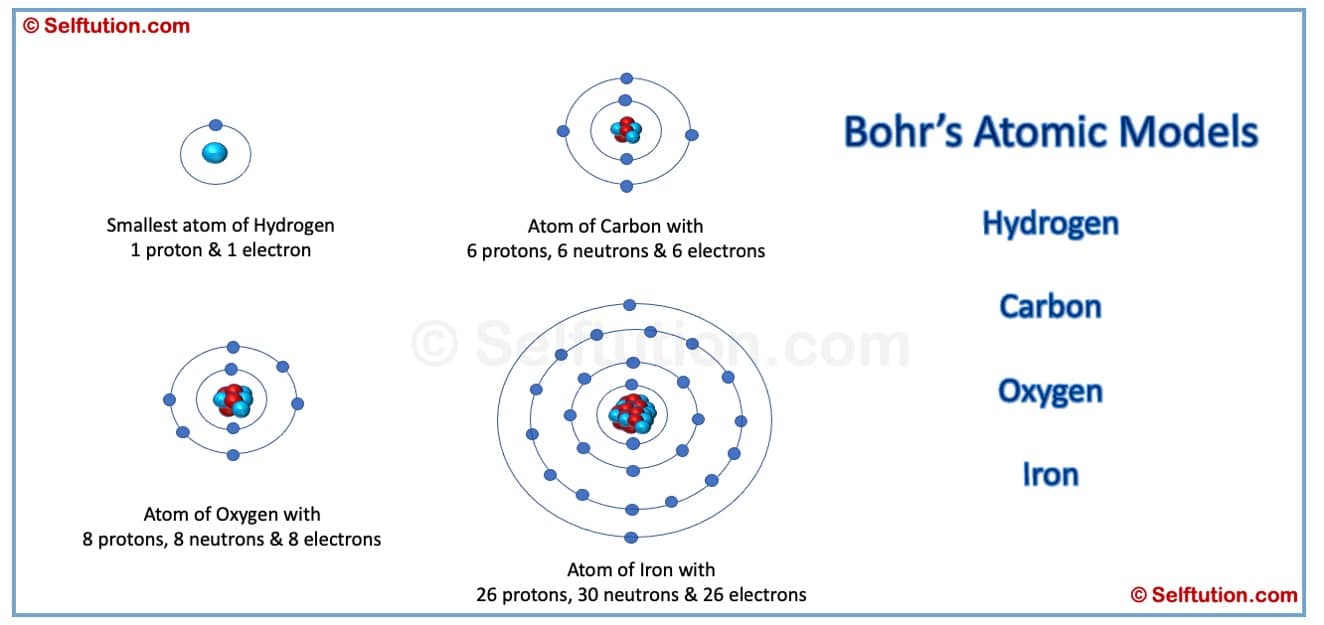
Bohr’s Atomic Model of Hydrogen, Carbon, Oxygen, and Iron
Application of Bohr’s Model to Hydrogen
Hydrogen is the simplest atom, consisting of one proton and one electron. According to Bohr’s model:
- The electron in a hydrogen atom moves in a circular orbit around the nucleus.
- In its lowest energy state, the electron stays in the first orbit (n=1, K-shell).
- If the atom absorbs energy, the electron jumps to a higher orbit (excited state).
- When the electron falls back to a lower energy level, it emits energy in the form of light.
- This emission of energy leads to the characteristic hydrogen spectrum seen as different colored lines.
Hydrogen Spectrum and Bohr’s Model
When hydrogen gas is heated or an electric current is passed through it, it emits light. If this light is passed through a prism, it splits into a series of colored lines known as the hydrogen spectrum.
These lines correspond to the energy differences between different orbits in Bohr’s model. The hydrogen spectrum consists of several series, including:
- Lyman Series: Ultraviolet region (electron falls to n=1)
- Balmer Series: Visible light region (electron falls to n=2)
- Paschen Series: Infrared region (electron falls to n=3)
Bohr’s model successfully explained why hydrogen emitted light in specific wavelengths and helped in understanding atomic structure.
Advantages of Bohr’s Model
Bohr’s atomic model was an improvement over earlier models and had several advantages:
- Explained the Stability of Atoms: It showed why electrons do not fall into the nucleus.
- Explained Atomic Spectra: It helped understand why atoms emit light at specific wavelengths.
- Introduced Quantum Concepts: It laid the foundation for modern quantum mechanics.
Limitations of Bohr’s Model
Despite its success, Bohr’s model had some limitations:
- Only Works for Hydrogen: It could not explain the spectra of larger atoms with more electrons.
- Did Not Explain Electron Behavior in Detail: It treated electrons as tiny balls moving in fixed orbits, which is not completely correct.
- Did Not Include Modern Quantum Mechanics: Later, scientists developed more advanced theories, such as the quantum mechanical model, which describes electron behavior more accurately.
Conclusion
Bohr’s atomic model for hydrogen was a groundbreaking step in understanding atomic structure. It explained how electrons move in fixed orbits, why atoms are stable, and how energy is absorbed or emitted. Although it had some limitations, it set the foundation for modern atomic physics. Today, we use more advanced models, but Bohr’s contributions remain an important part of science education.
By understanding Bohr’s model, students can gain a strong foundation in atomic physics, which helps in learning more advanced concepts in chemistry and physics.
You may also like…... Types of Chemical Reactions in Chemistry