Physical and Chemical Properties of Metals
Some basic physical and chemical properties of metals that differentiate them from non-metals and metalloids are as follows:
Physical Properties of Metals:
- Usually, metals are hard, opaque, possess luster, and conduct heat and electricity.
- They are malleable and ductile, can withstand longitudinal pull, and produce a resonant sound when struck.
- All metals are solid, except mercury, and gallium, which are liquids at room temperature.
- Metals usually have high melting and boiling points.
Chemical Properties of metals are:
- Metals possess 1, 2, or 3 valence electrons in the outermost shell of their atoms. Therefore, they lose or donate valence electrons to form positively charged ions (cations).
- They react with cold water, steam, and dilute acids to give hydrogen.
- Chlorides of metals are true salts and oxides of metals are usually basic.
INTRODUCTION
Before we proceed further with a detailed study of the physical and chemical properties of metals, let’s have a look at some common facts about them:
- There are three types of elements – metals, nonmetals, and metalloids. About 80% of the known elements in the periodic table are metals. To be precise, out of the 118 elements – 94 are metals, 17 are nonmetals and the remaining 7 are metalloids. However, this number may vary since the nature of some elements is still under research.
- The usage of metals by mankind predates ancient times as far as 3500 B.C. Artifacts made of copper and tin were discovered at excavation sites of the Indus Valley Civilization. The studies showed the use of iron by Asians in 2000 B.C. and later around 900-500 B.C. in China.
- Occurrence – Metals may occur in free and native states, for example, gold, silver, and platinum. However, most of them occur in a combined state as compounds along with earthly impurities. Metals are mined from the earth, but it does not offer them in their pure form for direct use. It gives us in the form of ores. The ore is a mineral from which we can extract metal economically. The process of extraction of metal from its ores is called metallurgy.
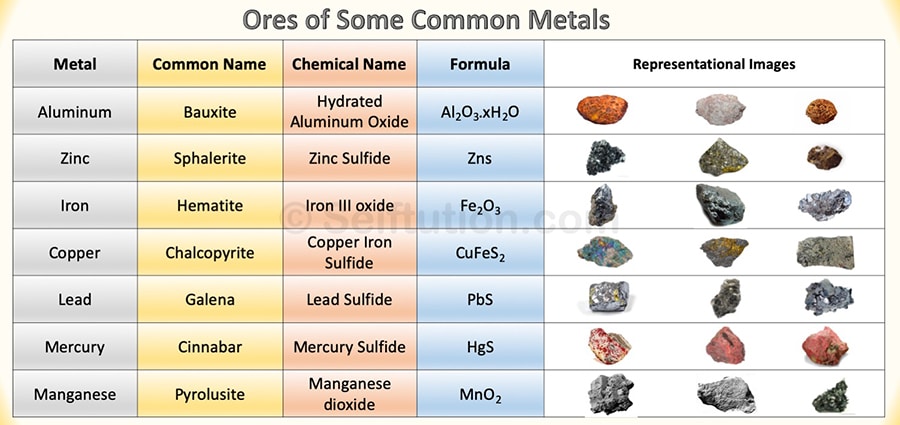
Ores of some common metals with chemical names and formulas
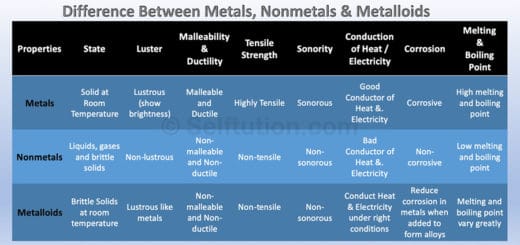
PHYSICAL AND CHEMICAL PROPERTIES OF METALS
Although every element has a distinct set of physical and chemical properties but still based on some common features, we can distinguish metals from nonmetals and metalloids. In this post, we will learn about various physical and chemical properties related to metals.
Physical Properties of Metals
Some common terms used for describing the physical properties of metals are – state, luster, hardness, malleability, ductility, tensile strength, sonority, conductivity, density, corrosivity, etc.

Physical Properties of Metals
Based on the physical properties, the uses of certain common metals are described below:
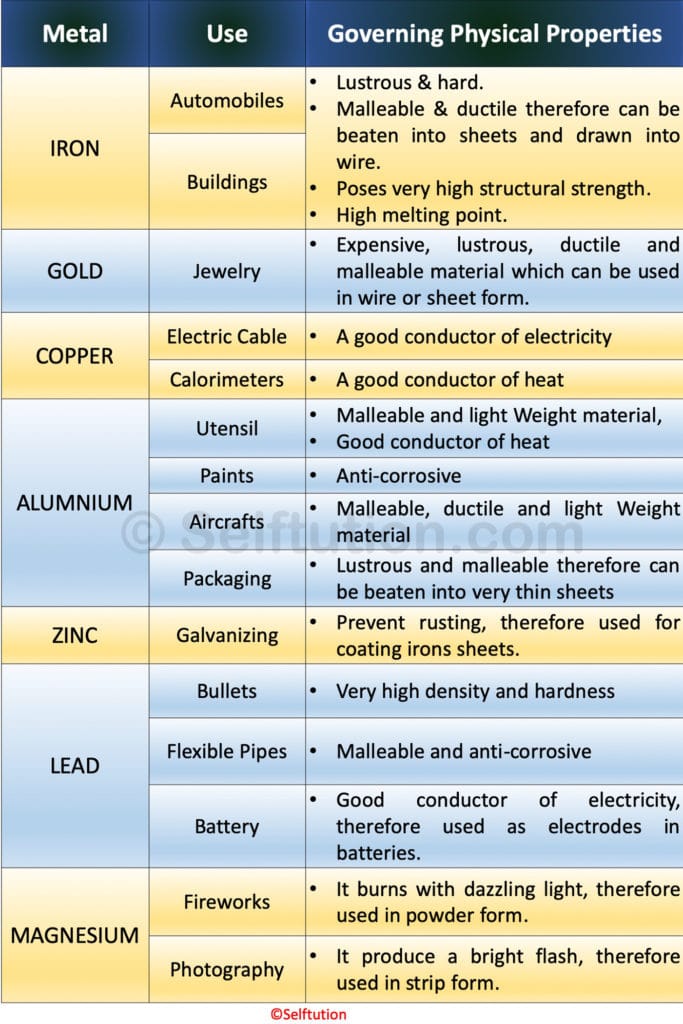
The table depicts certain uses of common metals based on their physical properties.
Chemical Properties of Metals
All metals react with common reagents like oxygen (air), hydrogen, halogens, sulfur, water, acids, etc. However, the extent of the reaction is different in the case of each metal.
Some of the chemical properties of metals are as follows:
- Metals react with oxygen present in the air to form oxides. These oxides dissolve in water to form an alkali.
- They form a range of salt when reacted with halogens (F, Cl, Br, I). For example, NaCl, KBr, etc.
- When reacted with cold water or steam metals release hydrogen and produce alkali as a by-product.
- Metals react with dilute acids to produce hydrogen gas and a salt solution.
Metals possess 1, 2, or 3 valence electrons in the outermost shell of their atoms. Therefore, they lose or donate valence electrons to form positively charged ions (cations). The chemical properties of metals depend upon their capability to lose electrons in the solution state to form positive ions. The more readily a metal loses its electrons, the more active it is, and the higher up it is in the reactivity series.
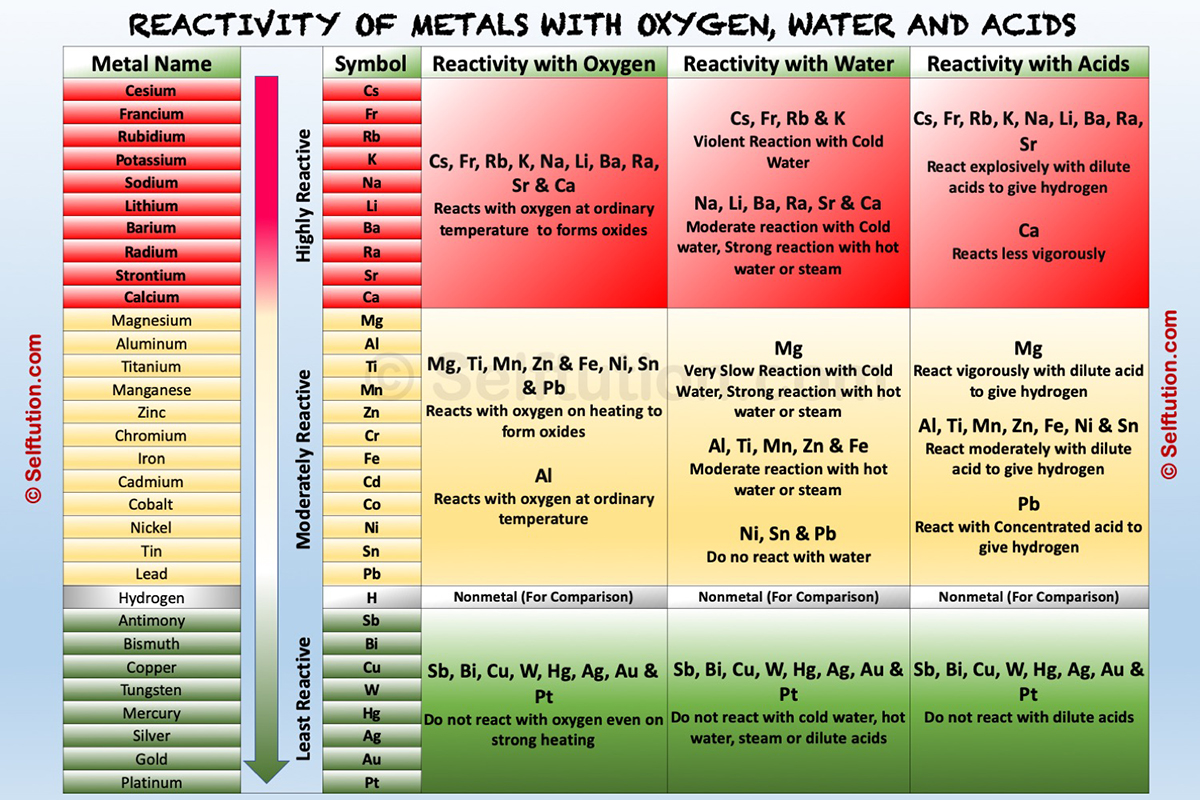
Reactivity Series of Metals
Metal reactivity series is a list in which metals are arranged in the decreasing order of their chemical activity.
Metal activity or reactivity series find its utility in the study of displacement reactions. It helps us to predict whether particular metal can displace another metal from a compound or not. In a displacement reaction, a metal higher up in the reactivity series displaces all other metals in a compound, which lies below it. For example, zinc being more active than copper replaces it with copper sulfate in a solution state to form zinc sulfate and free copper.
To understand the chemical properties of various metals, refer to the table below, which explains the reactions of metals with oxygen (air), water, dilute acids, and other salts solutions,
TYPES OF METALS
Based on different physical and chemical properties there are four types of metals – Base metals, Ferrous metals, Noble or Free metals, and Heavy metals.
- Base Metals – referred to metals that get oxidized easily. For example, iron, copper, nickel, aluminum, lead, zinc, tin, tungsten, molybdenum, tantalum, magnesium, cobalt, bismuth, cadmium, titanium, zirconium, antimony, manganese, beryllium, chromium, germanium, vanadium, gallium, hafnium, indium, niobium, rhenium, and thallium.
- Ferrous Metals – derived from the Latin word meaning ‘containing iron’. For example, pure iron, carbon steel, stainless steel, cast iron, alloy steel, etc.
- Noble or Free Metals – unlike base metals are resistant to corrosion and due to their rarity considered precious. There are 9 noble or free metals which are rhenium, ruthenium, rhodium, palladium, silver, osmium, iridium, platinum, and gold.
- Heavy Metals – possess a high density or atomic weight. Some examples of heavy metals are antimony, arsenic, bismuth, cadmium, cerium, chromium, cobalt, copper, gallium, gold, iron, lead, manganese, mercury, nickel, platinum, silver, tellurium, thallium, tin, uranium, vanadium, and zinc.
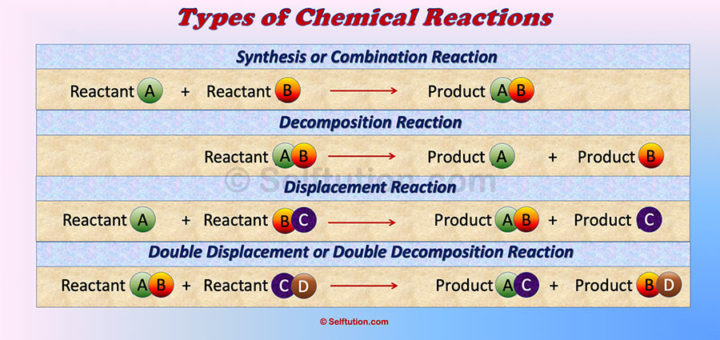
You may also like…...Types of Chemical Reactions in Chemistry
Back to the Physical and chemical properties of metals