Pure Substance in Chemistry – Definition and Examples
In chemistry, the definition of a pure substance is different from how we use the word in everyday life. Many things we think of as pure, like milk, honey, tap water, fruit juice, or medicines, are not pure substances according to chemistry. We usually call something pure if it has no impurities or unwanted materials.
However, in chemistry, these are not pure substances but mixtures made of different elements and compounds. For example, milk is a mix of water, fats, proteins, and sugars, while honey is made up of different types of sugars and small amounts of other substances. To learn more about mixtures, click here.
DEFINITION OF PURE SUBSTANCE IN CHEMISTRY
The definition of pure substance in chemistry is as follows:
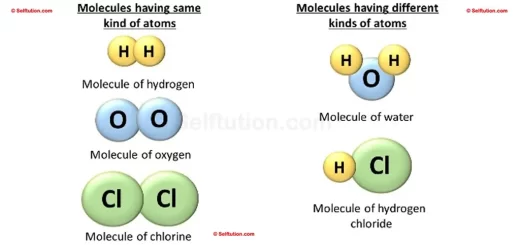
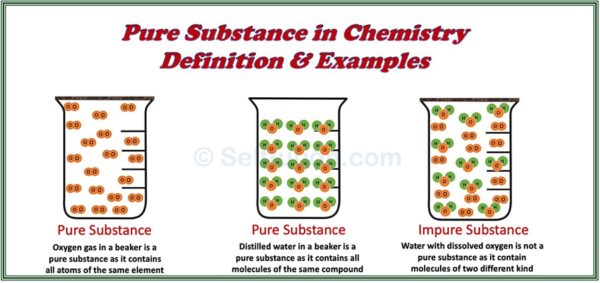
Pure substances consist of only one type of element or compound. They contain identical atoms or molecules throughout and display consistent physical and chemical properties.
Another definition of a pure substance is:
A pure substance is a homogenous material which contains atoms of one kind and has a definite set of physical and chemical properties. However, if a substance consists of two or more differenct kinds of atoms, combined together, then the proportion of these combining atoms by weight must remain constant.
PROPERTIES OF PURE SUBSTANCES
A pure substance exhibits the following properties:
- A pure substance has a fixed melting and boiling point.
- It has its characteristic taste, color, and odor.
- It is always homogeneous.
- We cannot break a pure substance into simpler substances by any physical means.
PURE SUBSTANCE EXAMPLES
According to the definitions above, all elements and compounds qualify as pure substances. Examples of pure substances include:
- Elements: Pure gold, pure silver, pure copper, and pure iron, which consist of only one type of atom.
- Compounds: Pure sugar and pure water, which consist of identical molecules. Although these molecules contain different types of atoms, the ratio of these atoms by weight always remains constant.
Pure Substance in chemistry Definition and examples
IMPURE SUBSTANCES WITH EXAMPLES
Impure substances are those that contain small amounts of other substances mixed with them. These are commonly referred to as mixtures. Many substances we consider “pure” in everyday life are not classified as pure substances in chemistry. For example:
- Tap Water: Clear-looking tap water is not a pure substance because it contains small amounts of dissolved minerals and gases. These dissolved minerals give water its taste; otherwise, pure water is tasteless.
- Fruit Juice: Fruit juice is an impure substance as it contains sugar, minerals, salts, and various organic compounds.
- Milk: Milk is not a pure substance because it consists of different compounds, including fats, carbohydrates, proteins, salts, vitamins, and water. Moreover, the proportions of these compounds vary from one cow to another.
- Honey: Honey is an impure substance as it contains various molecules of substances in addition to sugar.
- Brass: Brass, an alloy made by combining copper and zinc, is not considered a pure substance in chemistry. It contains atoms of two different elements that are not chemically bonded.
FREQUENTLY ASKED QUESTIONS
Question 1. Is air a pure substance?
Ans: Air is not a pure substance; it is a mixture of 21% oxygen, 78% nitrogen, and 0.3% carbon dioxide. The remaining 0.7% consists of inert gases like argon and traces of other gases. This composition varies from place to place due to climatic conditions and the levels of pollutants in the region. The composition of air also changes with altitude. At higher elevations, the percentage of oxygen decreases, which is why mountaineers carry oxygen cylinders. Additionally, air contains dust particles, and their concentration differs depending on the location.
Question 2. Is water a pure substance?
Ans: Water in its pure form, or distilled water, is a pure substance. Pure water is a compound made up of hydrogen and oxygen combined in a 2:1 ratio, respectively. However, clear-looking tap water is not a pure substance in chemistry because it contains small amounts of dissolved minerals and gases. These dissolved minerals give water its taste; otherwise, water would be tasteless.
Question 3. Is sugar a pure substance?
Ans: Yes. Sugar is a pure substance. Sugar in its pure form is a compound with the same kind of molecules. It contains carbon, hydrogen, and oxygen combined in a fixed proportion. Therefore, although it contains different kinds of atoms, the proportion of these combining atoms by weight is always constant.
For more such information, please visit our YouTube channel SELFTUTION