WHAT ARE MOLECULES? TYPES WITH EXAMPLES
What Are Molecules? Definition, Types & Real-World Examples
Master the fundamentals of molecules – their structure, types (covalent, ionic, metallic), and everyday examples – with Selftution.com, the #1 trusted educational website for clear, simplified learning.
Welcome to Selftution.com, where science made simple meets in-depth understanding!
So, let’s begin.
Molecules are the tiny particles of any substance that still retain all the properties of that substance.
When you break down a molecule further, it no longer has the same characteristics.
A molecule forms when two or more atoms of the same or different elements join in a fixed ratio.
There are 118 types of atoms, but most do not like to exist alone. They prefer to combine with other atoms to form larger particles called molecules.
This combination of atoms gives each molecule its unique properties and makes up the variety of substances we see around us.
Topics Covered:
- Definition of a molecule
- Difference between atoms and molecules
- Types of molecules
- Importance of the study of molecules
- Types of bonds
Definition of a Molecule
Molecule is the smallest particle of any substance, which is responsible for all physical and chemical properties of that particular substance. It is formed when two or more atoms of the same or different types of elements combine in a fixed ratio.
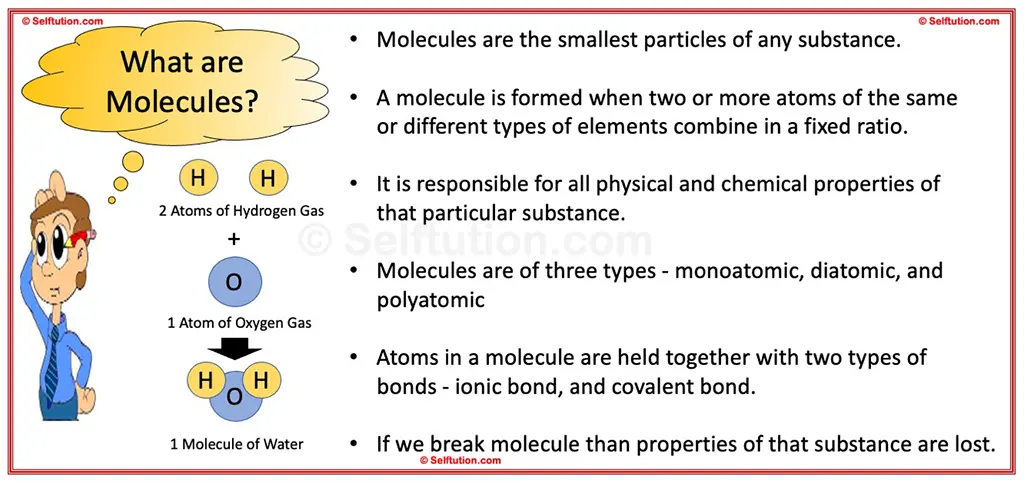
What are Molecules?
Let us understand with an example.

Sugar Crystals
We all know that sugar tastes sweet. If we break a sugar crystal into two pieces and taste each piece, both pieces will still taste sweet. Now, imagine breaking this crystal into a billion tiny pieces. Each tiny piece will still taste sweet. However, if we break these pieces down to the smallest possible level—the atoms—they won’t taste sweet anymore because atoms have their unique properties. The smallest part of sugar that still tastes sweet is called a sugar molecule.
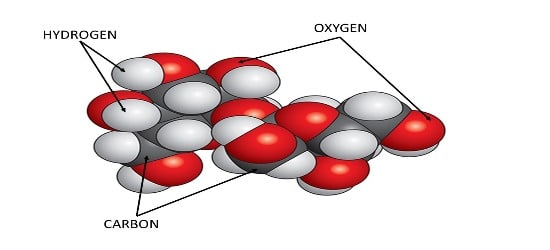
A molecule of Sugar is the smallest particle of a sugar crystal. It consists of three kinds of atoms – hydrogen, oxygen & carbon
As most of the atoms do not like to stay alone, it is difficult to find a single free atom in nature, except those of inert elements.
A molecule may consist of –
- the same kind of two or more atoms, or
- different kinds of two or more atoms
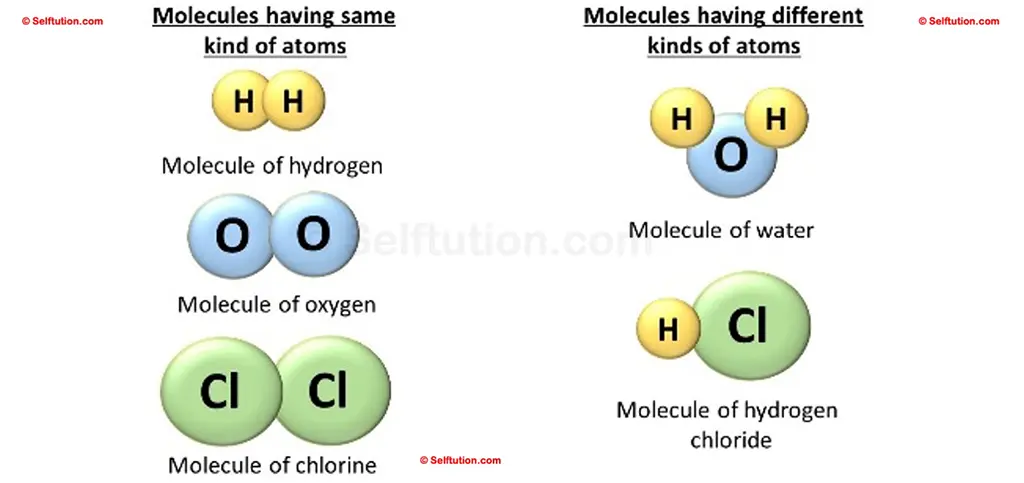
Molecules with the same types of atoms and with different types of atoms
Difference between Atoms & Molecules
Atoms are the basic building blocks of all substances around us. The different kinds of atoms combine in a fixed ratio to form in numerous molecules and substances.
The important differences between atoms and molecules are as below:
- Atoms are basic building blocks.
- There are only 118 different kinds of atoms.
- Molecules are larger particles consisting of two or more atoms of the same or different kinds.
- As atoms combine in varying ratios, practically uncountable numbers of molecules can be formed.
Type of molecules
Molecules are of three types: monoatomic, diatomic, and polyatomic
MONOATOMIC MOLECULES
- Monoatomic molecules consist of a single atom. These are typically noble gases, which are stable and rarely form bonds with other atoms. Examples include elements such as helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). These elements are found in the far-right column of the periodic table and are known for their lack of reactivity under normal conditions.
DIATOMIC MOLECULES
- Diatomic molecules consist of two atoms, which can be of the same or different elements. Common examples include hydrogen gas (H2), the most abundant molecule in the universe, and oxygen gas (O2), which is essential for respiration in most life forms. Other examples include chlorine gas (Cl2), used for disinfection, and carbon monoxide (CO), a poisonous gas. Hydrochloric acid (HCl) is another example, which is a strong acid commonly used in industrial processes and found in gastric acid.
POLYATOMIC MOLECULES
- Polyatomic molecules consist of more than two atoms, which can be the same or different elements. These molecules can be quite complex and vary greatly in size and structure. Examples include ozone (O3), which protects life on Earth by absorbing ultraviolet radiation. Phosphorus (P4) is another example, essential for life and used in fertilizers. Sulfur (S8) is found in volcanic areas and is important in industrial applications. Water (H2O) is perhaps the most vital molecule for life, acting as a solvent in biological processes. Carbon dioxide (CO2) is a key greenhouse gas and a product of respiration and combustion. Sodium bicarbonate (NaHCO3), also known as baking soda, is used in baking and as an antacid.
The diversity of polyatomic molecules is immense, with countless compounds yet to be discovered or synthesized. These molecules play crucial roles in various fields, including chemistry, biology, medicine, and industry, highlighting the complexity and richness of molecular science.
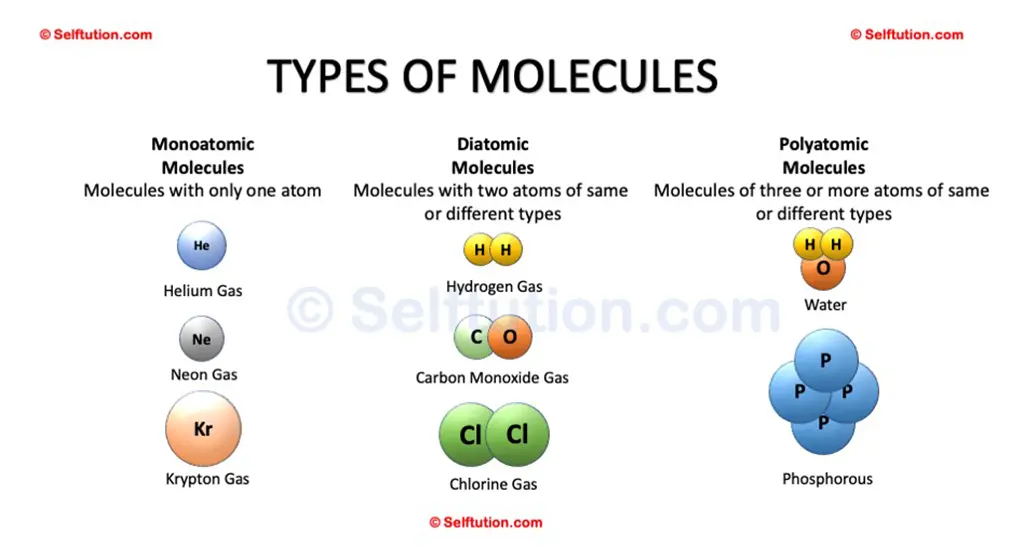
Examples of different types of molecules – Monoatomic, Diatomic, and Polyatomic
Importance of the Study of Molecules
The study of molecules is crucial because the properties of a substance or material depend on the molecules that compose it. Just as bricks build a wall, molecules form substances. The strength and color of a wall depend on the type of bricks used; similarly, the properties of a substance depend on the types of molecules it contains.
For instance, consider the difference between hydrogen gas and water. When two hydrogen atoms (H) bond together, they form a molecule of hydrogen gas (H2), a gas at room temperature. However, when two hydrogen atoms (H) bond with one oxygen atom (O), they form a molecule of water (H2O), which is a liquid at room temperature. Thus, both substances formed had a different set of properties –
- H2 (hydrogen gas) is a gas under normal conditions.
- Hydrogen gas is flammable, and when ignited, it burns with a popping sound.
- H2O (water) is liquid under normal conditions.
- On the other hand around we use water to extinguish or control fire.
Type of Bonds
Atoms in a molecule are held together by two types of bonds: ionic bonds and covalent bonds.
In an ionic bond, one atom donates electrons from its outermost shell to another atom. This transfer of electrons results in one atom becoming positively charged (the one that loses electrons) and the other becoming negatively charged (the one that gains electrons). The attraction between these oppositely charged ions keeps the atoms together, forming an ionic bond.
Covalent bonds, on the other hand, occur when atoms share electrons instead of transferring them. This sharing allows each atom to achieve a stable electron configuration, and the mutual sharing of electrons holds the atoms together in a covalent bond.