Interconversion of States of Matter With Examples
Interconversion of States of Matter: Solid, Liquid & Gas Explained with Real-World Examples | Selftution.com
Learn how matter changes states (melting, freezing, evaporation, condensation) through clear diagrams and everyday examples.
Welcome to Selftution.com – the best education website, simplifying science one concept at a time!
Matter can exist in four forms: solid, liquid, gas, and plasma. When matter changes its state from one form to the other, it is known as the interconversion of states of matter.
But before we proceed with the interconversion of the state of matter, let us learn what matter is and what the difference is between these four states of matter.
When you look around, you see many things like plants, animals, land, water, rocks, and various objects like desks, chairs, books, pens, bags, shoes, houses, and cars. Have you ever wondered what makes up all these things?
The answer is matter. Everything in the Universe, from the smallest particle of dust to the largest star, is made of matter.
Matter is not only what we can see or touch. It also includes things like the air we breathe and the perfume we smell. So, matter is all the physical substances that we can see, touch, smell, or feel around us.
Understanding matter and its states helps us comprehend the world better. Each state of matter has distinct properties that make it unique, and knowing these differences is key to studying how the interconversion of the states of matter occurs.
DEFINITION OF MATTER
The definition of matter for kids –
The matter is anything which occupies space and has a mass.
In the post “What is an Atom?” we discovered that atoms are the fundamental building blocks of everything around us. Interestingly, even atoms and subatomic particles themselves are composed of matter. This means that the smallest components, which make up everything we see and interact with, are also made of matter. Understanding this helps us appreciate the complexity and the fundamental nature of matter in the composition of the universe.
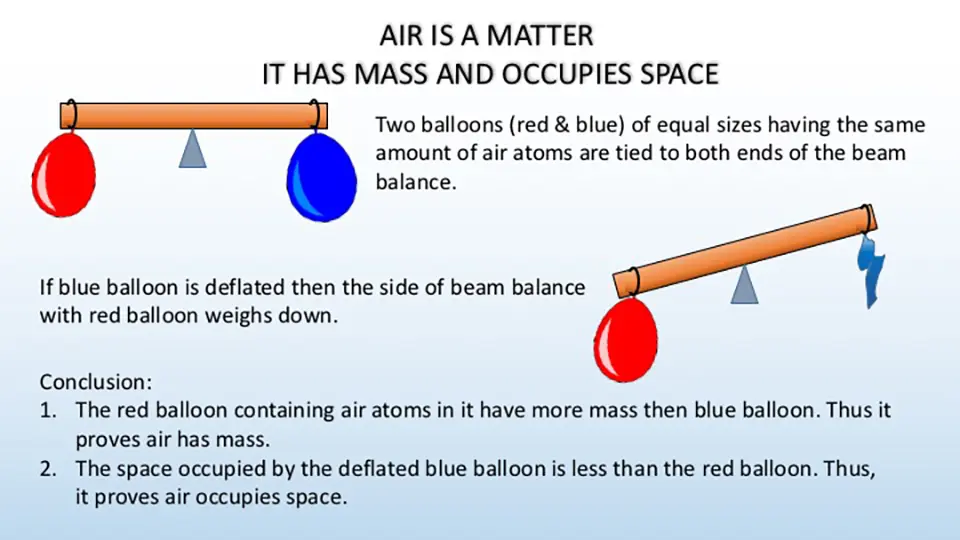
Experiment to demonstrate that air is a form of matter that occupies space and has mass
CAN MATTER BE CREATED?
The Universe consists of two fundamental components: matter and energy. Most scientists believe that matter and energy are interchangeable, each capable of converting into the other. During the Big Bang, which marked the creation of the Universe, an immense amount of energy was released. In just a few seconds, some of this energy transformed into tiny particles of matter. These particles combined to form atoms, which eventually made up the Universe we live in today. All the matter created during the Big Bang still exists, ranging in size from massive planets and stars to individual atoms and even subatomic particles. This matter exists in four states—solid, liquid, gas, and plasma—each capable of interconverting based on prevailing physical conditions. Notably, the interconversion of states occurs without altering the matter’s chemical composition.
FOUR STATES OF MATTERS
Since ancient times, people believed that matter existed only in three states or forms: solid, liquid, and gas. However, this understanding changed in the 1920s with the discovery of the fourth state of matter: plasma. Plasma is a very uncommon state that exists only at incredibly high temperatures, such as in nuclear reactors or inside stars.
Sometimes, the same substance can exist in three different states. For example, water is normally a liquid. If we pour it into an ice tray and place it in the freezer, it becomes solid (ice). When the same water is boiled, it changes into a gas (steam). Each state of matter has unique features and properties, but the atoms and molecules do not change. When a substance changes from one state to another without altering its chemical composition, we call it the interconversion of states of matter.
The different states of matter can be explained based on the arrangement of particles or molecules within them. Molecules within matter always attract each other with a force known as the intermolecular force. The force of attraction between two molecules increases as the space between them decreases, and decreases when the space between them increases. This space is referred to as intermolecular space.
The molecules of matter are always in random motion because they possess kinetic energy. The kinetic energy of these molecules increases with temperature and decreases when the temperature drops. Due to this random motion, molecules sometimes come closer together and sometimes move apart.
SOLIDS

In solids, the molecules are packed very closely together. There is a strong force of attraction between these molecules, and the space between them is minimal, almost negligible. Because of this close arrangement, the molecules are not free to move around but can only vibrate in their fixed positions. This tight packing of molecules makes solids hard and difficult to compress, which is why they maintain a fixed shape and size. This structural rigidity is a key characteristic that distinguishes solids from liquids and gases.
LIQUIDS
Molecules in liquids are less closely packed than in solids
In the case of liquids, the molecules are not as closely packed together as they are in solids. Additionally, the attraction between liquid molecules is not as strong as in solids. Consequently, there is more space between liquid molecules, allowing them to move about more freely. This fluidity enables liquids to flow and conform to the shape of the container into which they are poured. While liquids have fixed volumes, they do not possess a definite shape of their own.
GASES
Molecules in Gases are far away from each other
In the case of gases, the molecules exhibit minimal attraction towards each other. They are widely dispersed, with significant space separating them. The force of attraction between gas molecules is so weak that they enjoy considerable freedom of movement. Consequently, gases lack both a fixed shape and a fixed volume. They expand to fill the space available to them, adapting to the container’s shape. Because of the substantial space between gas molecules, gas can be readily compressed.
PLASMA

Cloud of Plasma
In contrast to the other three states of matter, plasma does not consist of atoms or molecules. It represents a distinct state, akin to gas, yet with properties that markedly diverge from those of gas. Within plasma, atoms shed electrons, resulting in a positive charge, while the liberated electrons move freely. This yields an electrically neutral gas wherein positively and negatively charged particles (ions) navigate freely within the same space. Plasma is an exceedingly rare state of matter, existing solely under extremely high temperatures, such as those found within nuclear reactors or in the cores of stars. Notably, plasma is not confined to Earth; it constitutes a significant portion of the Universe, comprising an estimated 99% of all observable matter. Its presence is pervasive in various celestial bodies, including stars, nebulae, and even interstellar space, where it manifests in diverse forms, contributing significantly to the cosmic landscape and dynamics.
COMPARISON BETWEEN PROPERTIES OF SOLIDS, LIQUIDS, GASES, AND PLASMA
The property-wise comparison between four states of matter, i.e., solid, liquid, gas, and plasma, is tabulated below:
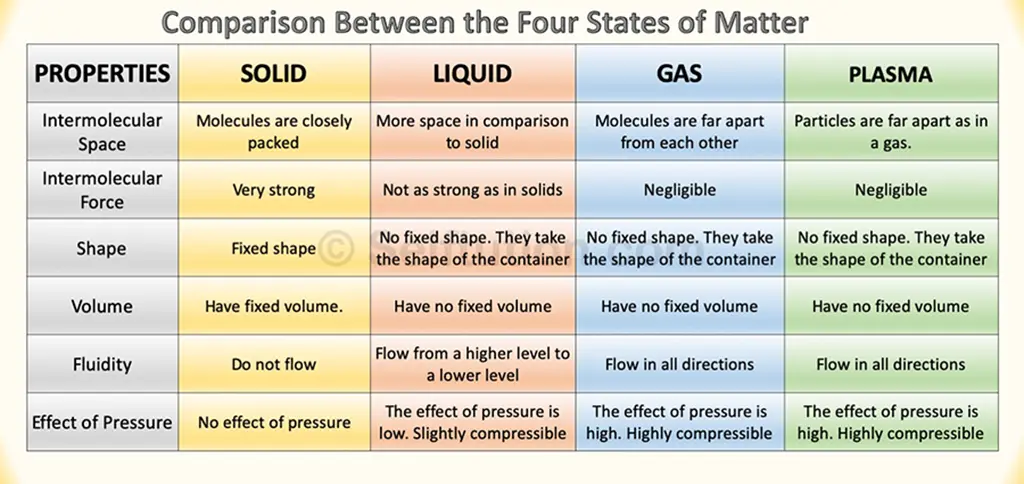
Comparison between the four states of matter
It is important to note that four states of matter – solid, liquid, gas, and plasma- undergo interconversion depending upon prevailing physical conditions.
INTERCONVERSION OF STATES OF MATTER
When matter changes its state from one form to another other it is known as the interconversion of states of matter.
The matter or substance can undergo state changes, transitioning from solid to liquid, liquid to gas, and vice versa. In everyday life, we encounter substances that undergo such transitions. For instance, water typically exists as a liquid, but when cooled, it transforms into ice. This occurs because cooling removes kinetic energy from water molecules in the form of heat, intensifying the force of attraction between them, causing them to come closer together and form solid ice.
Conversely, heating water causes it to boil and transition into steam. During boiling, heat provides kinetic energy to water molecules, weakening the intermolecular forces of attraction between them. Consequently, the molecules disperse further apart, resulting in the formation of steam, a gaseous state.
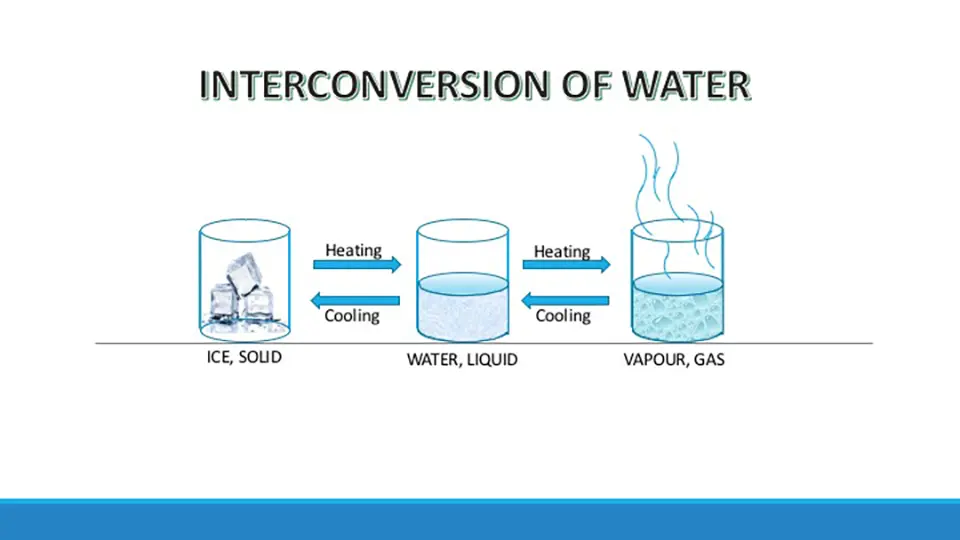
The picture depicts the interconversion of states of matter with an example of water. Here, water, a form of matter, undergoes interconversion from the solid to the liquid state and from the liquid to the gaseous state on heating. Similarly, on cooling, vapor changes to a liquid and further cooling, to a solid.
Video showing the interconversion of states of matter. Courtesy Amrita Vishwa Vidyapeetham University
SOME TERMS RELATED TO INTERCONVERSION OF STATES OF MATTER
- Melting or Fusion: It is a process by which a substance changes from a solid to a liquid state on heating.
- Melting Point: It is the temperature at which a pure substance changes from a solid to a liquid state.
- Vapourization or Evaporation: It is a process by which a substance changes from a liquid to a gaseous state on heating at any temperature.
- Boiling: It is a process by which a substance changes from a liquid to a gaseous state on heating at a fixed temperature.
- Boiling point: It is the temperature at which a pure substance changes from a liquid to a gaseous state.
- Condensation or liquefaction: It is a process by which a substance changes from a gas to a liquid state on cooling at a fixed temperature.
- Solidification or freezing: It is a process by which a substance changes from a liquid to a solid state on cooling