Difference Between Density and Viscosity
In science, many properties describe how materials behave. Two of these properties, density, and viscosity, are often confused because they relate to how substances interact with each other and their surroundings. However, these properties are very different.
Therefore, this blog explains density and viscosity in simple terms and highlights the key differences between them.
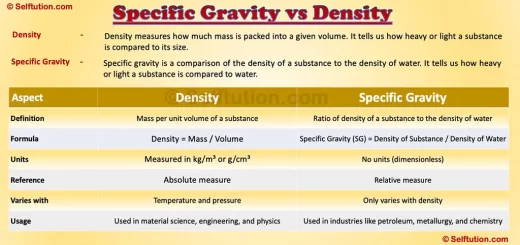
What is Density?
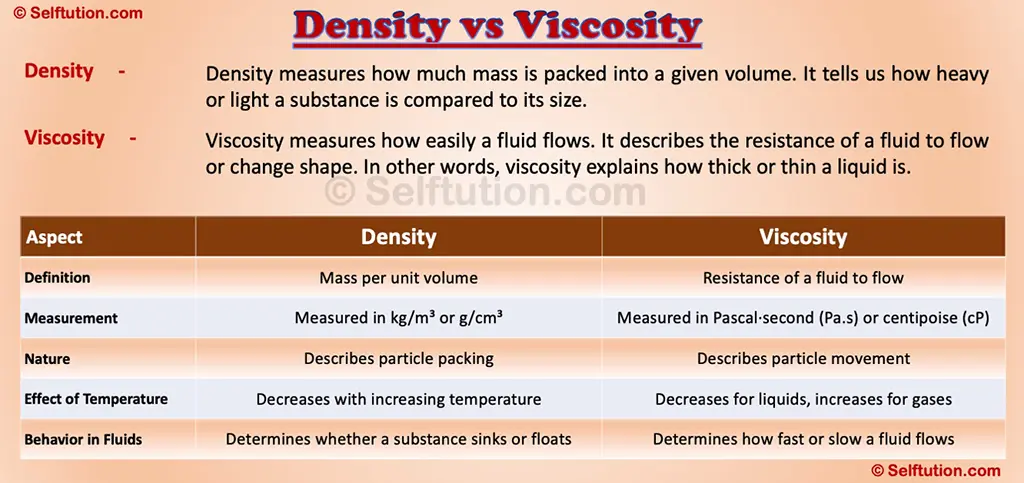
Density measures how much mass is packed into a given volume. It tells us how heavy or light a substance is compared to its size. Mathematically, density expresses the relationship using the formula:
Density (ρ) = Mass (m) / Volume (V)
The unit of density is usually expressed in kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³).
To visualize the concept, consider a container filled with different substances. A small amount of a heavy material like lead weighs much more than the same volume of a light material like foam. This difference in how closely the particles are packed explains why some substances feel heavier than others, even when they occupy the same space. Understanding this property helps determine whether an object will float or sink when placed in a liquid.

In practical applications, scientists and engineers rely on these measurements to design structures and develop new materials. For instance, determining the density of construction materials ensures that buildings can withstand stress without adding unnecessary weight. Similarly, in aviation, choosing lightweight materials helps maintain fuel efficiency and improves aircraft performance.
Understanding Density with Examples
- Water: The density of water is 1 g/cm³ or 1000 kg/m³. This means 1 cubic centimeter of water contains 1 gram of mass.
- Iron: The density of iron is about 7.87 g/cm³. This means 1 cubic centimeter of water contains 7.87 grams of mass. Consequently, iron is denser than water, which is why an iron object sinks in water.
- Oil: The density of oil is lower than that of water, which is why oil floats on water.
Factors Affecting Density
- Temperature: Increasing temperature usually decreases density because most substances expand when heated.
- Pressure: Increasing pressure generally increases the density of gases, as particles move closer together.
- Composition: Mixtures of substances may have different densities depending on their composition.
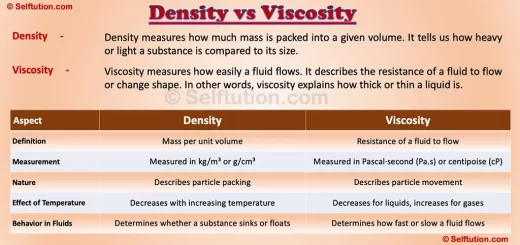
What is Viscosity?
Viscosity measures how easily a fluid flows. It describes the resistance of a fluid to flow or change shape. In other words, viscosity explains how thick or thin a liquid is.
Viscosity is usually measured in pascal-seconds (Pa·s) or centipoise (cP).
Understanding Viscosity with Examples
- Honey: Honey is thick and flows slowly. It has high viscosity.
- Water: Water is thin and flows quickly. It has a low viscosity.
- Motor Oil: Motor oil has a moderate viscosity, which changes with temperature. As a result, it becomes thinner at higher temperatures.
Factors Affecting Viscosity

Factors Affecting Viscosity
- Temperature: Higher temperatures reduce resistance to flow, making fluids move faster. As the particles in a liquid gain energy, they move more freely, which reduces the internal friction between them. Conversely, lower temperatures increase resistance to flow, making fluids thicker and slower.
- Pressure: While pressure has little effect on the resistance of liquids, it significantly impacts gases. Increasing pressure brings gas molecules closer together, making it harder for them to move past each other, which increases internal friction.
- Composition: The type and size of molecules affect the ease of flow. Substances with larger or more complex molecules often experience greater internal friction, making them flow more slowly. Additionally, the strength of molecular interactions, such as hydrogen bonding, can influence how freely particles move, affecting the overall resistance to flow.
- Shear Rate: Some fluids change their behavior when subjected to different forces. In non-Newtonian fluids, resistance to flow may decrease or increase depending on the applied force. For example, ketchup becomes thinner when shaken, while cornstarch solutions become thicker under stress.
- Additives and Impurities: The presence of additional substances in a liquid can alter its resistance to flow. Additives like polymers in lubricants can modify how the liquid behaves under varying conditions, ensuring optimal performance. Impurities in fluids can also affect their behavior by introducing irregularities in the molecular structure, impacting overall flow characteristics.
Key Differences Between Density and Viscosity
How Density and Viscosity Work Together
In real-life scenarios, density and viscosity often work together to affect the behavior of fluids. For example:
- Oil and Water: Oil is less dense than water, so it floats. However, oil is more viscous than water, which is why it flows more slowly.
- Lava Flow: Lava has both high density and high viscosity, which causes it to move slowly down a slope.
- Air and Fog: Air has low viscosity and low density, allowing it to flow easily, while fog, with its denser particles, moves differently.
Applications of Density and Viscosity
Density Applications
- Ships and Submarines: Ships float because designers ensure their average density is less than that of water. Similarly, submarines adjust their density by taking in or releasing water to float or sink. Additionally, ballast tanks in submarines control their depth by adjusting their density.
- Weather Patterns: Air density affects weather and climate. Warm air, being less dense, rises, while cool, denser air sinks. This movement of air creates wind patterns and influences weather conditions globally. Meteorologists study air density variations to predict changes in the atmosphere.
- Material Selection: Engineers choose materials based on their density to ensure strength and stability in structures. For example, aerospace engineers use lightweight materials with low density to build aircraft, while construction engineers use denser materials like concrete and steel for durability and stability.
- Buoyancy in Fluids: Objects float or sink depending on their density relative to the surrounding fluid. This principle applies to designing life jackets, which keep people afloat by reducing overall density. Similarly, hot air balloons rise because the air inside them becomes less dense when heated.
Viscosity Applications
- Lubricants: Engine oils need the right viscosity to reduce friction and wear in machines. Lubricants with appropriate viscosity ensure smooth operation by forming a protective film between moving parts. Engineers carefully select lubricants with viscosity that changes minimally with temperature fluctuations.
- Food Industry: Understanding viscosity helps in designing products like sauces and syrups to achieve the desired consistency. Food manufacturers control viscosity to ensure that products like ketchup pour smoothly while maintaining thickness.
- Pharmaceuticals: Viscosity affects the flow of liquids in medical applications, such as intravenous fluids. Pharmaceutical companies design liquid medications with appropriate resistance to flow to ensure easy administration and absorption in the body. Additionally, the viscosity of gels and creams determines their effectiveness and ease of application.
- Paints and Coatings: Paints and coatings must have the correct viscosity to ensure smooth application and even coverage. High-viscosity paints may be difficult to spread, while low-viscosity paints may drip excessively.
Misconceptions About Density and Viscosity
- High Density Means High Viscosity: Not true. Mercury is very dense but has low viscosity, while honey is less dense but highly viscous.
- Viscosity Affects Sinking or Floating: Viscosity influences flow, not whether an object will sink or float. Instead, density determines the sinking or floating of an object.
Summary
In summary, density and viscosity are distinct but important properties. Density measures how much mass is packed into a given space, while viscosity measures how resistant a fluid is to flow. As a result, understanding these differences helps explain how substances behave in various environments. Moreover, knowing how density and viscosity interact also plays a critical role in industries ranging from engineering and aviation to food and healthcare.
By grasping the concepts of density and viscosity, students can better understand the physical world and how materials behave under different conditions.
You may also like…... Types of Chemical Reactions in Chemistry