Rutherford Atomic Model and Gold Foil Experiment
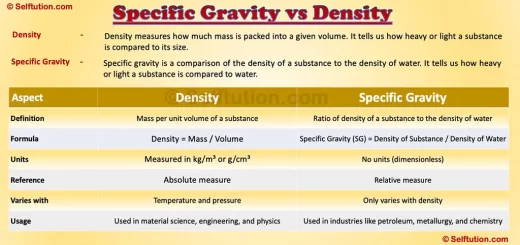
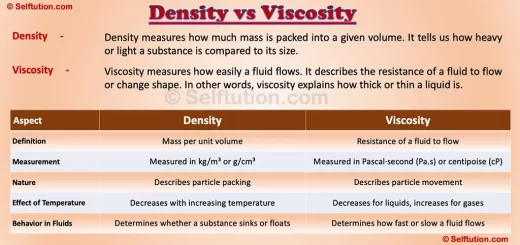
The Rutherford atomic model transformed the scientific understanding of the atom. Ernest Rutherford developed it in 1911 through his revolutionary gold foil experiment, also known as the alpha particle scattering experiment.
By bombarding a thin gold foil of thickness 4 micrometers with alpha particles, Rutherford aimed to study the arrangement of electrons and positively charged particles in an atom. The experiment led to groundbreaking conclusions about atomic structure.
The Rutherford atomic model revealed that atoms have a small, dense, positively charged core, later called the nucleus.
Surprisingly, Rutherford’s research paper never mentioned the term “nucleus.” Despite this, he remains celebrated worldwide for unveiling the atomic nucleus, a discovery that transformed physics and chemistry.
Rutherford’s Gold Foil Experiment or the Alpha Particles Scattering Experiment
Ernest Rutherford (30 August 1871 – 19 October 1937)

Lord Ernest Rutherford, the one who discovered the nucleus and protons
Ernest Rutherford was a New Zealand-born British physicist. His full name was Ernest, Baron Rutherford of Nelson. Due to his immense contribution to the study of radioactivity and atoms, he is also known as the father of nuclear physics. Rutherford won the Nobel Prize for Chemistry in 1908 and served as president of the Royal Society for the period of nearly five years from the year 1925 to 1930. His most famous work was when he overturned the plum-pudding atomic model of J. J. Thomson based on the results of the gold foil or the alpha particles scattering experiment.
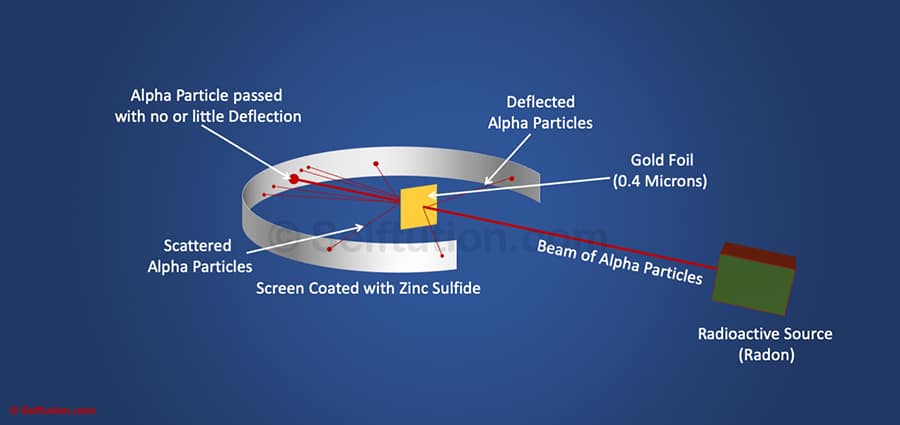
Rutherford Gold Foil or Alpha Particles Scattering Experiment
Alpha particles are positively charged particles comprising two numbers of protons and two numbers of neutrons. In other words, they are helium atoms without two numbers of electrons. Some atoms of heavy elements with a large neutron-to-proton ratio in their nuclei emit alpha particles to bring parent nuclei to a more stable configuration.
Rutherford discovered the nucleus in an experiment widely known as the gold foil experiment. In his experiment, Rutherford surrounded a thin sheet or foil of gold (0.00004 cm thickness) with a screen made of Zinc Sulfide. The screen allowed passage for a thin beam of alpha particles emitted from the radioactive source. The radioactive source used by Rutherford for emitting alpha particles was Radon. He bombarded this thin foil of gold with alpha particles in an evacuated chamber. Alpha particles, after reflecting or passing through the gold sheet, struck the Zinc Sulfide screen and produced a red glow at the point of contact.
Observations of the Gold Foil or the Alpha Particles Scattering Experiment
Rutherford noted locations where alpha particles struck the screen and observed as follows:
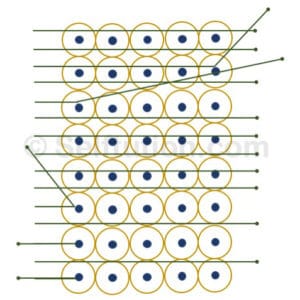
Gold Foil bombarded with Alpha Particles during the Experiment
- Most of the alpha particles passed straight through the foil without any deflection from their path.
- A small fraction of them was deflected from their original path by small angles.
- Only a few particles bounced back.
Step-by-step demonstration of the Alpha particles scattering experiment. Video courtesy blenderIITB
Conclusions of the Gold Foil or the Alpha Particles Scattering Experiment
Based on the above observations made during the alpha particle scattering experiment, Rutherford made the following conclusions to develop his atomic model:
- Most of the space in an atom was empty because alpha particles went straight.
- The deflection of a small fraction of alpha particles occurs due to the presence of a heavy positively charged mass in the atom.
- The positively charged mass is very small and centrally located because only a few particles bounce back. He named this positively charged mass discovered by him – the nucleus of an atom.
Rutherford Atomic Model
Based on his gold foil experiment, Rutherford suggested an atomic model. According to this model, an atom consists mainly of two parts:
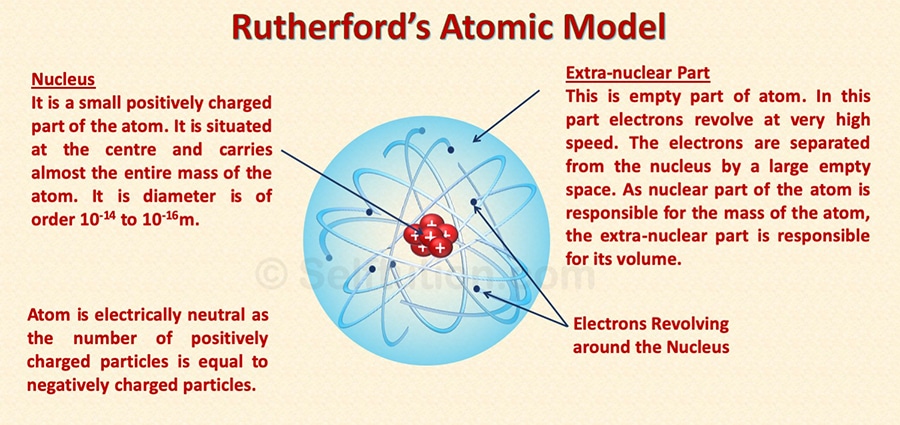
Part 1: The centrally located nucleus
- The nucleus is a centrally located positively charged mass.
- As electrons have negligible mass, thus, the entire mass of the atom is present inside the nucleus. It is the densest part of the atom.
- If compared to the size of the atom as a whole, the size of the nucleus is very small.
If we consider a circular stadium as an atom, then its nucleus is no more than a cricket ball placed at the center of the stadium.
Part 2: The outer circular orbits
- Electrons revolve around circular orbits (shells) in the space available around the nucleus.
- An atom is electrically neutral, i.e., the number of protons and the number of electrons present in an atom are equal.
Thus, a model proposed by Rutherford is somewhat similar to that of the solar system. Just as in the solar system, the sun is at the center and the planets revolve around it, in an atom, the electrons revolve around the centrally located nucleus containing protons. Therefore, some people also consider Rutherford’s atomic model a planetary model of an atom. However, it is not correct. Niels Bohr, a Danish physicist, presented the actual planetary model in the year 1915.
Rutherford discovered the proton in 1917, much later after the discovery of the nucleus. He discovered that positively charged particles present in the nucleus are similar to those of H+ ions and named them ‘protons’.
Frequently Asked Questions on the Rutherford Atomic Model
Q1. Why did Rutherford use a gold foil in this experiment?
Ans. Rutherford used gold foil in his experiment because, of all metals known to mankind, gold is the most malleable. As he wanted his foil to be as thin as possible, therefore, to achieve the same he therefore used gold.
Q2. What is the thickness of the gold foil used by Rutherford?
Ans. The gold foil used by Rutherford in his experiment was about 4 micrometers, i.e., 2410 atoms thick. Please note that the diameter of a single gold atom is 1.44 x 10-10 m.
Q3. Why did Rutherford use alpha particles in his experiment?
Ans. Some of the important reasons for the use of alpha particles by Rutherford in the gold foil experiment are:
- Rutherford discovered alpha particles in the year 1899.
- Alpha particles are easily available. Heavy elements such as uranium, radium, polonium, and radon emit alpha particles during radioactive decay.
- Detection of alpha particles was easy. They produce a flash of light on striking a screen coated with zinc sulfide.
- Alpha particles are helium atoms without electrons. Therefore, they are doubly charged particles of mass 4 amu. The alpha particles move at a very high speed with a considerable amount of energy, which is sufficient to penetrate through the thin gold foil used in the experiment.
- The health effects of direct exposure to alpha particles are not a major concern. Although alpha particles are very energetic, they are so heavy that they are unable to travel very far from the atom. They lack the energy to penetrate even the outer layer of human skin.