Who Discovered Electrons? – The Cathode Ray Experiment
J.J. Thomson, an English scientist, discovered electrons in 1897, challenging the long-held belief that atoms were indivisible.
Before Thomson’s groundbreaking work, scientists thought that atoms were the smallest unit of matter. However, during his experiments with cathode rays, Thomson discovered that atoms contain negatively charged subatomic particles called electrons, proving that atoms are indeed divisible.
Following Thomson’s discovery of electrons, a race ensued among scientists to further explore the fundamental structure of the atom. This quest led to the discovery of other subatomic particles, such as protons and neutrons, ultimately reshaping our understanding of atomic structure.
To learn about the basic structure of an atom, click here
Who Discovered Electrons
CATHODE RAY EXPERIMENT

J. J. Thomson, the one who discovered electrons
Scientists in the early nineteenth century were aware of electricity and the effect of electric potential. The electric potential is a driving force that results in the flow of current through a substance due to the difference in concentration of charges at two ends of it. The magnitude of the electric current flowing through a substance is directly proportional to the electric potential applied across it. They proved that electricity or the electric current can flow through any substance – solid, liquid, or gas if enough driving force or electric potential exists. After conducting a series of experiments with solids, liquids, and gases, scientists took one step further to drive electricity through a vacuum. However, most of them failed as none of them were able to create a perfect vacuum.
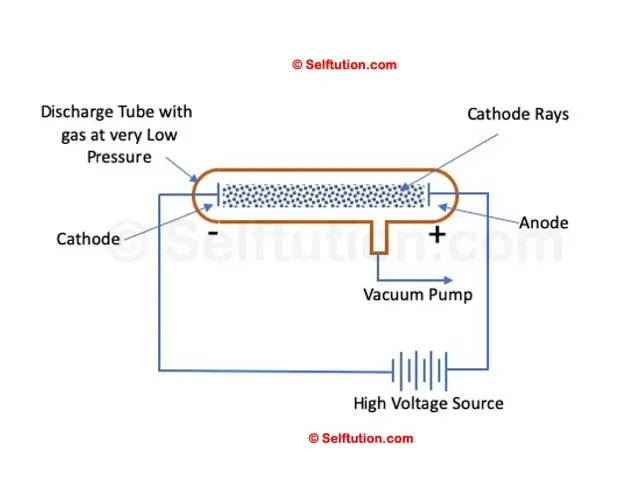
Simple Setup of William’s Cathode Ray Experiment
In 1875, British scientist William Crookes successfully created a near-perfect vacuum (0.01 mm of mercury) in a glass tube sealed at both ends with metal plates, which he named the discharge tube. Crookes observed that when a high electric potential was applied across the discharge tube, a current flowed from the negative terminal (cathode) to the positive terminal (anode) in the form of rays, which he called ‘cathode rays.’ However, Crookes was unable to determine what these cathode rays were made of or what exactly was moving from the negative terminal to the positive terminal.

William Crook is the first person to pass an electric current through a vacuum
Finally, in 1897, J. J Thomson discovered electrons while studying the characteristics of cathode rays. He discovered that cathode rays consist of negatively charged subatomic particles (now called electrons), present in all atoms of the elements.
DISCOVERY OF ELECTRONS – THOMSON EXPERIMENT AND RESULT
In his experiments, J.J. Thomson applied an electric field across the path of cathode rays in the discharge tube. He observed that the cathode rays were deflected towards the positive plate of the electric field, indicating that they consisted of negatively charged particles. Next, Thomson introduced a magnetic field in the path of the cathode rays and observed that they deflected in a direction consistent with the behavior of moving negative charges. This further confirmed that cathode rays contained negatively charged particles, which he named electrons.
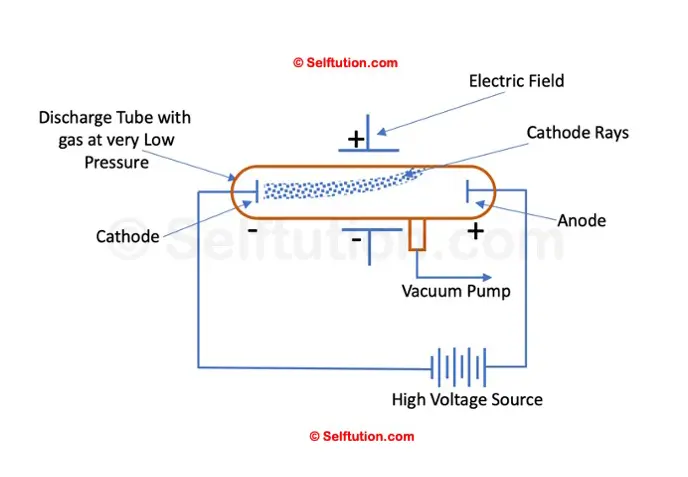
Deflection of cathode rays toward the positive plate proves that they consist of negatively charged particles
For demonstration, refer to this interesting video by the CBC Demo lab at OSU
Thomson noted that the deflection of cathode rays increased with the strength of the electric or magnetic field. Using this observation, he measured the mass-to-charge ratio (m/q) of particles in the cathode rays. He found that this ratio was constant regardless of the gas in the discharge tube. Thus, he proved that cathode rays contained particles of the same type. However, Thomson discovered electrons but couldn’t measure their mass (m) or charge (q) separately.
The exact mass of the electron was determined later, in 1911. American scientist Robert Andrews Millikan successfully calculated the minimum charge (q) that any particle could carry through his oil drop experiment.

Robert Andrews Millikan is the one who discovered the value of the charge on electrons
SYMBOL AND PROPERTIES OF AN ELECTRON
The symbol used to denote an electron is -1e0. The superscript ‘0’ represents its mass, and the subscript ‘-1’ represents its one-unit negative electrical charge.
- Electrons are an integral part of all atoms.
- An Electron has a definite mass, and it carries a definite electric charge.
- The mass of an electron is 1/1837 of the mass of a hydrogen atom (9.108 x 10-28 g).
- It bears one unit negative charge, which is equal to 1.602 x 10-19 Coulombs.
THOMSON MODEL OF AN ATOM – PLUM PUDDING MODEL
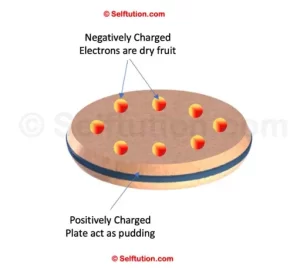
Thomsons Plum-Pudding Model
After J.J. Thomson discovered negatively charged electrons in 1897, German scientist Eugen Goldstein concluded that since atoms are electrically neutral, there must also be positively charged particles within them. He discovered these positively charged particles in 1898 through experiments with a modified discharge tube.
In 1904, J.J. Thomson proposed the first model of the atom, known as the Plum Pudding Model. In this model, the atom is a positively charged sphere. Electrons are embedded within it, similar to dry fruits in a pudding. Since the atom’s total positive charge equals the total negative charge of electrons, the model suggested that gaining electrons would make an atom negatively charged. Losing electrons would make it positively charged. However, this model failed to explain many experimental observations about atoms.