Who Discovered Proton And The Nucleus?
Who Discovered the Proton and the Nucleus? – Key Scientists, Experiments & Breakthroughs
Uncover the groundbreaking discoveries of the proton and atomic nucleus – from Rutherford’s gold foil experiment to Chadwick’s findings.
At Selftution.com, we make complex science simple, accurate, and engaging. Welcome to the #1 educational website for clear, in-depth learning!
So, let’s begin.
Eugen Goldstein, a German, discovered the presence of positive particles in the year 1898, and Ernest Rutherford, a New Zealand-born British, discovered the nucleus in 1911 and the proton in 1917.
TOPICS COVERED:
Who Discovered the Proton
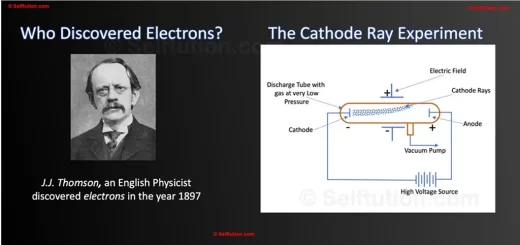
The discovery of the electron by J.J. Thomson in the year 1897 revealed to the world that atoms are divisible. This motivated other scientists and led to the discovery of the nucleus and other subatomic particles – the proton and neutron.
After J.J. Thomson discovered negatively charged electrons, Eugen Goldstein, a German, concluded that since all atoms are electrically neutral, there must be positively charged particles present in them. He discovered positively charged particles in the year 1898 while experimenting with a discharge tube, although with slight variation.
Eugen Goldstein discovered anode rays
Some of the people around the world mistakenly consider Eugen Goldstein as the one who discovered the proton. But, it is not correct. He discovered anode rays, which comprise positively charged particles that may consist of particles other than a proton. It was Ernest Rutherford who discovered the proton in the year 1917. Nevertheless, the work of Eugen Goldstein could not be undermined, as protons would not have been discovered if positively charged particles had not been discovered in the first place.
Eugen Goldstein’s Experiment and Result
Goldstein experimented with a discharge tube fitted with a perforated cathode and a layer of Zinc Sulphide applied on the wall of the glass tube behind the cathode.
On applying a high electric potential across the discharge tube, he noted the presence of another ray along with cathode rays. These rays were traveling as waves in the opposite direction, i.e., from the anode to the cathode. Some of these rays pass through holes in the cathode and result in a red glow after striking the glass wall covered with Zinc Sulphide. He named them ‘anode rays’.
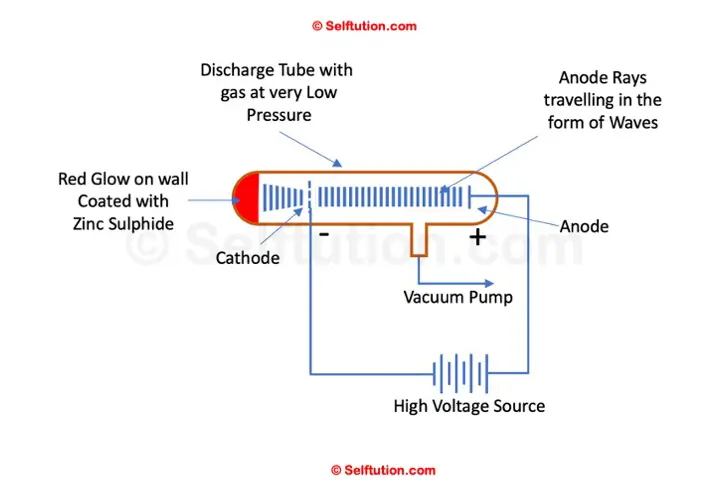
Goldstein Anode Ray Experimental Setup
The rays or waves emitted from the anode behave just the opposite of the cathode ray in all respects when subjected to an electric and magnetic field. This proved that anode rays contained positively charged particles. Goldstein successfully measured the mass-to-charge (m/q) ratio of the particles comprising anode rays. He also noted that this ratio of mass to charge (m/q) varies with the type of gas inside the discharge tube. Thus, it proved that anode rays comprise particles of varying types.
Later on, Ernest Rutherford in the year 1911 conducted an alpha particle scattering experiment to uncover the basic structure of an atom. He discovered that all the positively charged particles join together to form a ‘nucleus’. This nucleus is present at the center of an atom, and all electrons revolve around it.
In the year 1917, he discovered that these positively charged particles present in the nucleus are similar to those of H+ ions and named them ‘protons’.
Symbol & Properties of Porton
The symbol used to denote a proton is +1p1, where the superscript ‘1’ represents 1 AMU (atomic mass unit) mass and the subscript ‘+1’ represents its one unit positive charge.
- A proton is an integral part of the nucleus of all atoms.
- A proton has a definite mass, and it carries a definite electric charge.
- The mass of a proton is nearly equal to the mass of an atom of hydrogen, i.e., 1.672 x 10-24 g.
- The positive charge on a proton is equal to the negative charge on an electron, i.e., 1.602 x 10-19 coulombs.
Who Discovered the Nucleus
In 1911, Ernest Rutherford, a scientist from New Zealand, experimented to find the arrangement of electrons and positively charged particles, i.e., protons, in an atom. His experiment led to the discovery of a small, positively charged nucleus at the center of an atom.
Rutherford Alpha Particle Scattering Experiment
Alpha particles are positively charged particles comprising two numbers of protons and two numbers of neutrons. In other words, they are helium atoms without two numbers of electrons. Some atoms of heavy elements with a large neutron-to-proton ratio in their nuclei emit alpha particles to bring parent nuclei to a more stable configuration.
The nucleus was discovered by Rutherford in an experiment widely known as the alpha particle scattering experiment. In his experiment, Rutherford surrounded a thin sheet of gold (0.00004 cm thickness) with a screen made of Zinc sulfide. The screen allowed passage for a thin beam of alpha particles emitted from the radioactive source. He bombarded this thin sheet of gold with alpha particles in an evacuated chamber. Alpha particles, after reflecting or passing through the gold sheet, struck the Zinc sulfide screen and produced a red glow at the point of contact. He noted locations where alpha particles struck the screen and concluded as follows:
- Most of the alpha particles passed straight through the foil without any deflection from their path.
- A small fraction of them was deflected from their original path by small angles.
- Only a few particles bounced back.
Step-by-step demonstration of the Alpha particles scattering experiment. Video courtesy blenderIITB
Based on the above observations, Rutherford made the following conclusions:
- Most of the space in an atom was empty because alpha particles went straight.
- The deflection of a small fraction of alpha particles occurs due to the presence of a heavy positively charged mass in the atom.
- The positively charged mass is very small and centrally located because only a few particles bounced back. He named this positively charged mass discovered by him, the ‘nucleus’ of an atom.
Rutherford Atomic Model
Based on his experiment, he suggested a model for the structure of the atom. According to this model, an atom consists mainly of two parts:
Part 1: The centrally located nucleus

Ernest Rutherford, the one who discovered the nucleus and protons
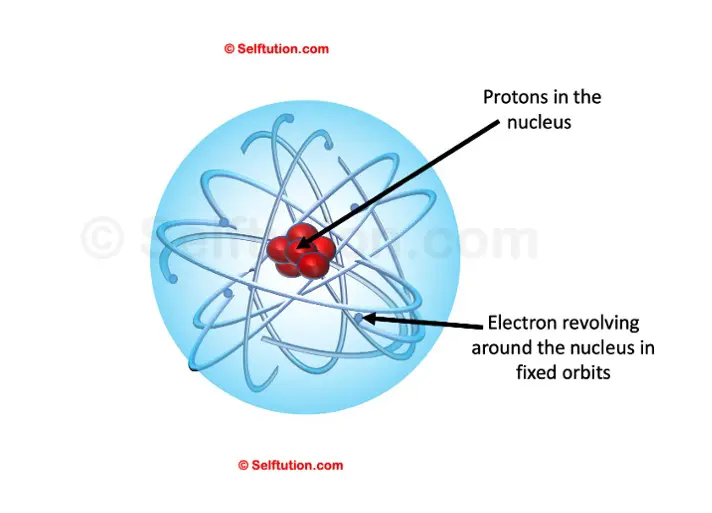
- The nucleus is a centrally located positively charged mass.
- As electrons have negligible mass, the entire mass of the atom is present inside the nucleus. It is the densest part of the atom.
- If compared to the size of the atom as a whole, the size of the nucleus is very small.
If we consider a circular stadium as an atom, then its nucleus is no more than a cricket ball placed at the center of the stadium.
Part 2: The outer circular orbits
- Electrons revolve around circular orbits (shells) in the space available around the nucleus.
- An atom is electrically neutral, i.e., the number of protons and the number of electrons present in an atom are equal.

Rutherford’s Atomic Model of an atom
Thus, a model proposed by Rutherford is somewhat similar to that of the solar system. Just as in the solar system, the sun is at the center and the planets revolve around it, in an atom, the electrons revolve around the centrally located nucleus containing protons.
Rutherford discovered the proton in 1917, much later after the discovery of the nucleus. He discovered that positively charged particles present in the nucleus are similar to those of H+ ions and named them ‘protons’.
FREQUENTLY ASKED QUESTIONS
Q1. Who discovered the proton?
Ans: Ernest Rutherford, a New Zealand-born British discovered the proton.
Q2. In which year was Proton discovered?
Ans: Ernest Rutherford discovered the proton in 1917.
Q3. What was discovered by Eugen Goldstein?
Ans: Eugen Goldstein discovered anode rays, which comprise positively charged particles that may consist of particles other than a proton.
Q4. Who discovered the nucleus?
Ans: Ernest Rutherford discovered the nucleus.
Q5. In which year was the nucleus discovered?
Ans: Ernest Rutherford discovered the nucleus in 1911 when he carried out the ‘Alpha Particle Scattering Experiment’.