Who Discovered Atoms – Indian, Greek or English?
Most people credit the discovery of atoms to either English chemist John Dalton (1766-1844 AD) or ancient Greek philosopher Democritus (470-400 BC). However, few know that an Indian philosopher discovered atoms first.
The great Indian sage Maharishi Kanad discovered atoms in 600 BC. He called them “paramanus,” with “param” meaning ultimate and “anu” meaning particle.
Since ancient times, thinkers like Maharishi Kanad, Democritus, and John Dalton have suspected that everything is made of tiny particles. However, without experimental proof, their atomic theories were based solely on logical and scientific reasoning.
Today, with advances in science, we know that atoms are real. We can even see atoms using very powerful electron microscopes.
Topics Covered:
Related Topics:
ATOMIC THEORY OF MAHARISHI KANAD

Maharishi Kanada
Maharishi Kanad, an Indian sage, presented his atomic theory in his book “Kanada Sutras“. He discovered that all matter consists of indestructible particles called paramanus, which we now call atoms. According to Kanad, these paramanus do not exist independently but combine with other paramanus to form larger particles called anus, which we now know as molecules.
He explained that there are different types of paramanus, each with its specific properties. Kanad’s idea was revolutionary for his time, as he proposed that everything in the universe consists of these tiny, indestructible particles. His theories were based on logical and scientific thinking rather than experimental proof, as the technology to observe atoms did not exist during his era.
Kanad’s atomic theory was significant because it laid the foundation for later scientific discoveries about the nature of matter. While it took centuries for these ideas to be experimentally confirmed, Kanad’s work was an early and important step toward our modern understanding of atomic theory. His insights into the composition of matter highlight the advanced level of scientific thought in ancient India.
To learn more about Maharishi Kanad, click here.
DEMOCRITUS ATOMIC THEORY

Democritus, an ancient Greek philosopher from around 470-400 BC, proposed that everything in the World consists of tiny, invisible particles. He believed these particles were incredibly hard, eternal, and always in motion. He named these particles “atoms,” derived from the Greek word “atomos,” meaning indivisible. Democritus’s atomic theory suggested that atoms are the fundamental building blocks of all matter.
In the Western world, Democritus is often credited with discovering atoms. However, Maharishi Kanad, an Indian sage, discovered atoms much earlier, around 600 BC. Nevertheless, it is important to note that no evidence suggests that Kanad’s discoveries influenced Democritus’s work. Democritus’s theory was based on logical reasoning and laid important groundwork for future scientific advancements, even though the technology to prove the existence of atoms did not exist in his time.
JOHN DALTON’S ATOMIC THEORY OR MODEL
John Dalton (1766-1844 AD) was a science teacher and meteorologist who formulated the atomic model based on his observations and experiments. He proposed that every element comprises of tiny, indivisible particles called atoms. Dalton discovered that atoms of the same element are identical in mass and properties but differ from atoms of other elements. He contributed to chemistry by assigning symbols to 36 elements based on their atomic weights.
Dalton’s atomic model depicted atoms as solid spheres, akin to tiny, indestructible balls. However, further scientific advancements later revealed that atoms are not indivisible and can indeed be split into subatomic particles. Additionally, some substances that Dalton classified as elements were later identified as compounds or combinations of elements.
Despite these refinements over time, Dalton’s atomic theory laid a foundational framework for understanding the structure and behavior of matter, marking a significant advancement in the field of chemistry.

John Dalton and his symbols for 36 elements
Key Features
The main features of John Dalton’s atomic theory or model are:
- Matter consists of tiny and indivisible particles called atoms.
- The atoms of an element are identical in all respects i.e., size, mass, density, and chemical properties, but they differ from the atoms of other elements.
- Atoms of an element combine in small numbers to form molecules of that element.
- Atoms of an element combine with the atoms of another element in a simple whole-number ratio to form molecules of compounds.
- An atom is the smallest unit of matter that takes part in a chemical reaction.
- Atoms can neither be created nor destroyed. During chemical reactions, the only rearrangement of atoms takes place.
Due to various experimental studies, we now know that most of the features of atoms as described by John Dalton’s atomic theory are incorrect. Even though Dalton was right that atoms take part in chemical reactions.
Similarities in Maharishi Kanad, Democritus, and Dalton’s Atomic Theories or Models
Although Maharishi Kanad, Democritus, and John Dalton discovered atoms with significant gaps in time between them, their atomic theories share several similarities. They all proposed that:
- Atoms are indestructible.
- An atom is the smallest indivisible particle of an element.
- Atoms of the same element are identical in all respects.
Difference between Dalton’s and Modern Atomic Theories or Models
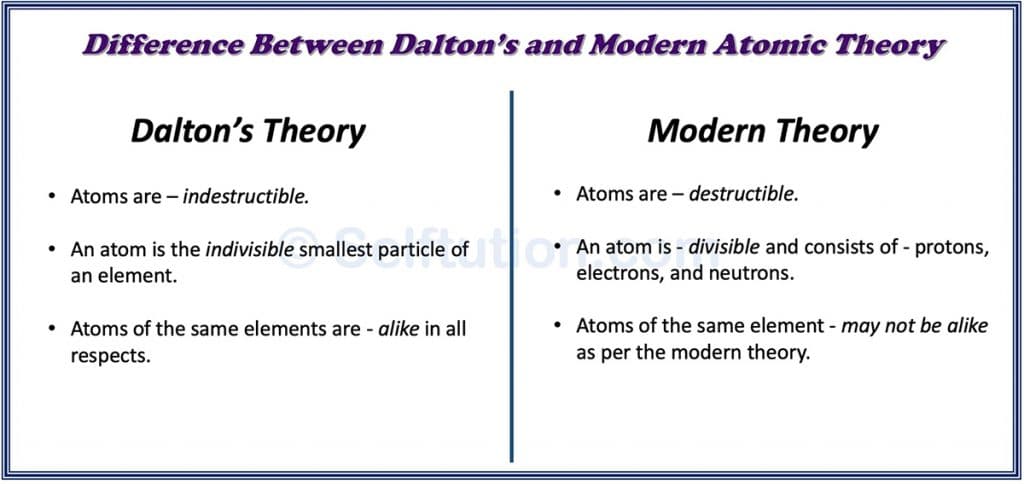
Difference between Dalton’s and Modern Atomic Theories