Who Discovered Electrons, Protons and Neutrons?
Who Discovered Electrons, Protons, and Neutrons? – The Complete Breakthrough Story
Selftution.com | The #1 Trusted Educational Website for Simplified Science
Explore the groundbreaking discoveries of electrons (J.J. Thomson), protons (Ernest Rutherford), and neutrons (James Chadwick) with clear timelines, diagrams, and interactive explanations.
Welcome to Selftution.com – where complex science becomes effortless learning!
So, let’s begin.
Most people around the world credit the following four scientists as those who discovered electrons, protons, neutrons, and the nucleus:
- J.J. Thomson, an English physicist, discovered electrons in 1897.
- Eugen Goldstein, a German physicist, discovered the presence of positive particles in 1898.
- Ernest Rutherford, a New Zealand-born British physicist, discovered the nucleus in 1911 and protons in 1917.
- James Chadwick, an English physicist, discovered neutrons in 1932.
TOPICS COVERED:
1.0 DISCOVERY OF ELECTRONS
Who discovered electrons?

J. J. Thomson, the one who discovered electrons
For a long time, scientists believed that atoms were indivisible. This view changed in 1897 with the groundbreaking discovery by English scientist J.J. Thomson. While studying the properties of cathode rays, Thomson discovered that atoms contain negatively charged subatomic particles called electrons, proving that atoms are indeed divisible.
Following Thomson’s discovery of electrons, scientists raced to uncover the atom’s basic structure. This pursuit led to the discovery of the nucleus and other subatomic particles, namely protons and neutrons.
1.1 Cathode Rays
In the early nineteenth century, scientists were aware of electricity and the effects of electric potential. The electric potential is a driving force that results in the flow of current through a substance due to the difference in concentration of charges at two ends of it. The magnitude of the electric current flowing through a substance is directly proportional to the electric potential applied across it. They demonstrated that electric current could flow through any solid, liquid, or gas substance if a sufficient driving force or electric potential were applied. After conducting numerous experiments with these states of matter, scientists attempted to drive electricity through a vacuum. However, most failed because they could not create a perfect vacuum.
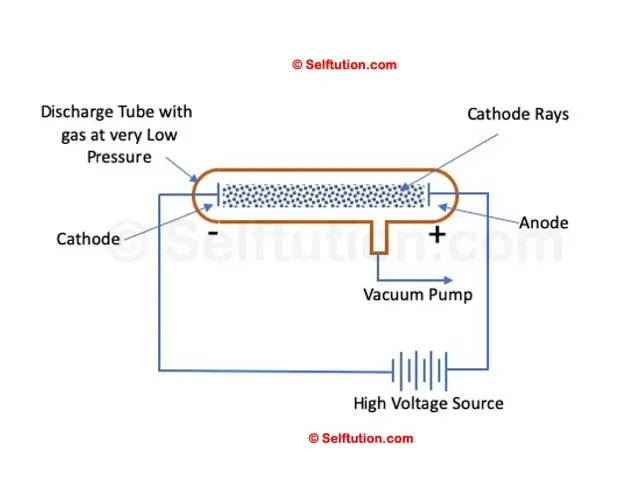
Simple Setup of William’s Cathode Ray Experiment

William Crook is the first person to pass an electric current through a vacuum
In 1875, British scientist William Crookes successfully created a near-perfect vacuum (0.01 mm of mercury) in a glass tube sealed at both ends with metal plates. He named this apparatus the discharge tube. Crookes observed that when a high electric potential was applied across the discharge tube, current flowed from the negative terminal to the positive terminal in the form of rays, which he called ‘cathode rays’. However, he was unable to determine the composition of the electric current or cathode rays, nor what was moving from the negative to the positive terminal.
Finally, in 1897, J.J. Thomson discovered electrons while studying the characteristics of cathode rays. He found that cathode rays consisted of negatively charged subatomic particles, now known as electrons, which are present in all atoms of the elements.
1.2 Thomson’s Experiment and Result
Discovery of electrons
In his experiment, J.J. Thomson applied an electric field across the path of cathode rays in a discharge tube. He observed that the cathode rays were deflected towards the positive plate of the electric field, indicating that they consisted of negatively charged particles.
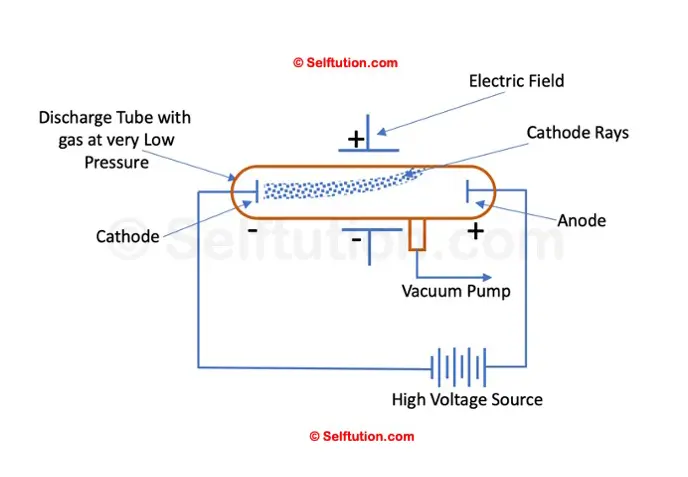
Deflection of cathode rays toward the positive plate proves that they consist of negatively charged particles
Next, he applied a magnetic field in the path of the cathode rays and noted that the rays were deflected in a direction consistent with the behavior of moving negative charges. This confirmed that cathode rays contained negatively charged particles, which Thomson identified as electrons.
This video beautifully demonstrates the effect of a magnetic field on cathode rays. Courtesy kosasihiskandarsjah
Thomson also observed that the degree of deflection of the cathode rays was directly proportional to the strength of the magnetic or electric field applied. Using this principle, he measured the mass-to-charge ratio (m/q) of cathode rays particles. He noted that this ratio remained constant regardless of the gas in the discharge tube, proving that cathode rays consisted of the same particles. Although Thomson discovered electrons, he failed to measure their mass (m) or charge (q) separately.
The exact mass of electrons was determined in 1911 through the groundbreaking oil-drop experiment by American scientist Robert Andrews Millikan. His experiment successfully calculated the minimum charge (q) that could be carried by any particle, establishing a foundational milestone in understanding atomic structure.
1.3 Symbol & Properties of Electron
An electron is denoted by the symbol -1e0. The superscript 0 represents its mass and the subscript -1 represent it’s one unit negative electrical charge. The mass of an electron is 1/1837 of the mass of a hydrogen atom (9.108 x 10-28 g) and its charge is one unit negative charge i.e. 1.602 x 10-19 Coulombs
Back to who discovered electrons
2.0 DISCOVERY OF PROTONS
Who discovered protons?
Eugen Goldstein discovered anode rays
After the discovery of electrons in 1897, Eugen Goldstein reached a significant conclusion in 1898: since all atoms are electrically neutral, they must contain positively charged particles. He observed these positively charged particles, known as anode rays, during an experiment with a discharge tube, albeit under slightly different conditions.
It’s a common misconception that Eugen Goldstein discovered protons. However, he identified anode rays, which consist of positively charged particles that may or may not include protons.
It was Ernest Rutherford who definitively discovered protons in 1917.
2.1 Eugen Goldstein’s Experiment and Result
Discovery of positively charged particles
Goldstein experimented using a discharge tube equipped with a perforated cathode and coated with Zinc sulfide on the glass tube wall behind the cathode.
When a high electric potential was applied across the discharge tube, Goldstein observed the emergence of rays in addition to cathode rays. These rays traveled in waves in the opposite direction—from the anode to the cathode. Some of these rays passed through the holes in the cathode and produced a red glow upon striking the Zinc Sulphide-coated glass wall. Goldstein termed these rays ‘anode rays’. Unlike cathode rays, the rays emitted from the anode exhibited behavior opposite in response to electric and magnetic fields, confirming their composition of positively charged particles.
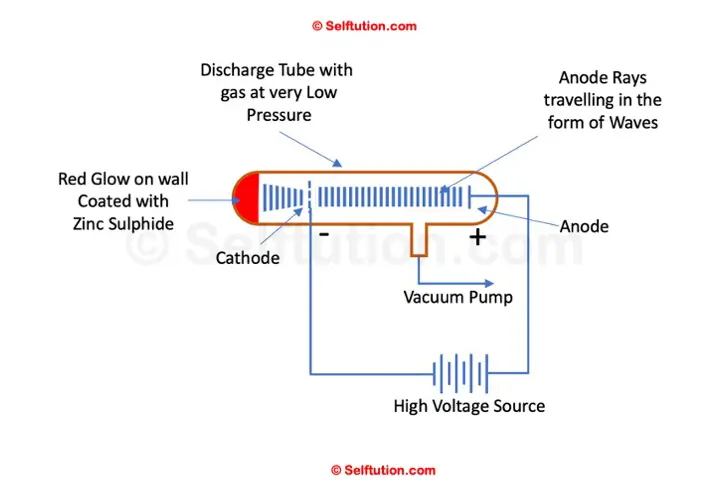
Goldstein Anode Ray Experimental Setup
Goldstein successfully determined the mass-to-charge ratio (m/q) of the particles comprising anode rays. He also noted that this ratio varied with the type of gas inside the discharge tube, indicating the presence of different particles within anode rays.
In 1911, Ernest Rutherford conducted the famous alpha particle scattering experiment to unravel the basic structure of an atom. He discovered that all positively charged particles gathered to form a central ‘nucleus’ within the atom, around which electrons orbited.
In 1917, Rutherford identified these positively charged particles as similar to H+ ions and named them ‘protons’.
2.2 Symbol & Properties of Porton
A proton is denoted as +1p1, where the superscript ‘1’ represents 1 amu (atomic mass unit) mass and the subscript ‘+1’ represent it’s one unit positive charge. The mass of a proton is equal to the mass of an atom of hydrogen, i.e. 1.672 x 10-24 g. The positive charge on a proton is equal to the negative charge on an electron, i.e. 1.602 x 10-19 coulombs.
Back to who discovered electrons
3.0 THOMSON MODEL OF AN ATOM – PLUM PUDDING MODEL
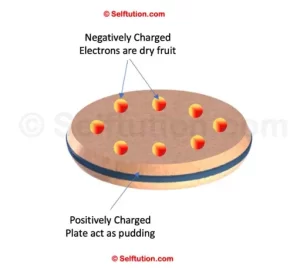
Thomsons Plum-Pudding Model
In 1904, J.J. Thomson developed the first model of an atom, the Plum Pudding Model.
According to this model, an atom is a positively charged sphere with electrons embedded within it, similar to how dry fruits are distributed throughout a pudding. Therefore, it is called the Plum Pudding Model.
Thomson proposed that the total positive and negative charges in an atom are equal. Consequently, this implies that an atom would become negatively charged if it gains electrons and positively charged if it loses electrons. However, despite its innovative approach, this model failed to explain many experimental observations about atoms. As a result, it was eventually replaced by more accurate atomic models.
Back to who discovered electrons
Back to who discovered protons
4.0 DISCOVERY OF THE NUCLEUS
In 1911, Ernest Rutherford, a scientist from New Zealand, experimented to find the arrangement of electrons and protons in an atom. His experiment led to the discovery of a small, positively charged nucleus at the center of an atom.
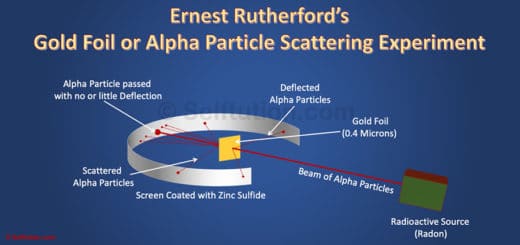
4.1 Rutherford Alpha Particle Scattering Experiment
Alpha particles are positively charged particles comprising two numbers of protons and two numbers of neutrons. In other words, they are helium atoms without two numbers of electrons. Some atoms of heavy elements with a large neutron-to-proton ratio in their nuclei emit alpha particles to bring parent nuclei to a more stable configuration.
Rutherford discovered the nucleus through the famous alpha particle scattering experiment. In this experiment, Rutherford used a thin sheet of gold (0.00004 cm thick) surrounded by a Zinc sulfide screen. This setup allowed a thin beam of alpha particles emitted from a radioactive source to pass through. He bombarded the gold sheet with alpha particles in an evacuated chamber. When the alpha particles struck the Zinc sulfide screen after reflecting off or passing through the gold sheet, they produced a red glow at the point of contact. By observing where the alpha particles hit the screen, Rutherford made several significant conclusions.
- First, he noted that most alpha particles passed straight through the foil without deflection from their path.
- Additionally, he observed that a small fraction of the particles were deflected from their original path by small angles.
- Lastly, he saw that only a few particles bounced back.
Step-by-step demonstration of the Alpha particles scattering experiment. Video courtesy blenderIITB
Observations:
Based on these observations, Rutherford concluded the following:
- Most of the space in an atom is empty, as evidenced by the fact that most alpha particles went straight through without deflection.
- The deflection of a small fraction of alpha particles occurred due to the presence of a heavy, positively charged mass within the atom.
- This positively charged mass is very small and centrally located since only a few particles bounce back upon collision.
Rutherford named this centrally located, positively charged mass the ‘nucleus‘ of the atom.
4.2 Rutherford Atomic Model
Based on his experiment, he suggested a model for the structure of the atom. According to this model, an atom consists mainly of two parts:
Part 1: The centrally located nucleus

Ernest Rutherford, the one who discovered the nucleus and protons
- The nucleus is a centrally located positively charged mass.
- As electrons have negligible mass, the entire mass of the atom is present inside the nucleus. It is the densest part of the atom.
- If compared to the size of the atom as a whole, the size of the nucleus is very small.
If we consider a circular stadium as an atom, then its nucleus is no more than a cricket ball placed at the center of the stadium.
Part 2: The outer circular orbits
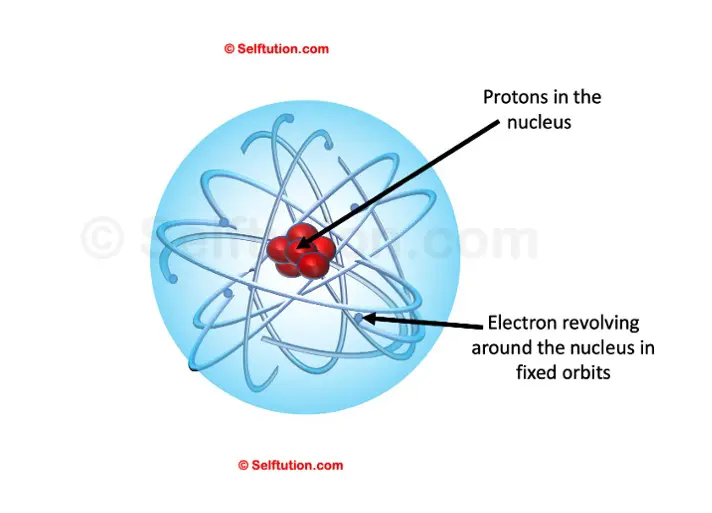
- Electrons revolve around circular orbits (shells) in the space available around the nucleus.
- An atom is electrically neutral i.e. the number of protons and the number of electrons present in an atom is equal.

Rutherford’s Atomic Model
Thus, a model proposed by Rutherford is somewhat similar to that of the solar system. Just as in a solar system, the sun is at the center and the planets revolve around it, in an atom, the electrons revolve around the centrally located nucleus containing protons.
5.0 DISCOVERY OF THE NEUTRONS

James Chadwick, the one who discovered Neutrons
James Chadwick in the year 1932, discovered neutrons. He studied the atomic model and found that the nucleus of the atom consists of particles with no charge a mass almost equal to that of protons. He named these newly discovered particles ‘neutrons’.
Until 1930, scientists believed that atoms consisted of only two types of subatomic particles: protons and electrons. Although the atomic model proposed by Rutherford answered most questions regarding the structure of an atom, some issues remained unresolved.
For example, since particles with similar charges repel each other, how can all positively charged protons stay together inside a nucleus? Furthermore, why is the mass of the nucleus greater than the total mass of the protons present within it? Additionally, why do some atoms of the same element have different masses?
Chadwick’s discovery of neutrons provided answers to these questions, significantly advancing the understanding of atomic structure.
5.1 Experiments Conducted Before James Chadwick
In 1930, scientists Walther Bothe and Herbert Becker conducted an experiment where they struck beryllium atoms with alpha particles. This experiment resulted in the emission of high-energy radiation. Observing the radiation’s high penetrating power and lack of ionization of the gas through which it passed, they concluded that the radiation was composed of gamma rays.
However, two years later, Curie and Joliot conducted further experiments that challenged this conclusion. They bombarded a hydrogen-rich target with this high-energy radiation and observed the ejection of high-energy protons. Given that gamma rays do not possess the necessary energy and momentum to displace protons, the findings of Curie and Joliot directly contradicted the earlier conclusions made by Bothe and Becker.
This discrepancy highlighted the need for further investigation and ultimately led to a deeper understanding of the nature of the radiation emitted in these experiments.
5.2 James Chadwick’s Experiment and Result
In 1932, James Chadwick conducted a series of experiments using paraffin wax and the radiation identified by Bothe and Becker, leading to the discovery of neutrons. He chose paraffin wax because it is hydrocarbon-rich in hydrogen and, consequently, in protons. By measuring the energy of the displaced protons, Chadwick concluded that gamma rays alone could not account for the observed energy and momentum. Therefore, he deduced that the radiation emitted from beryllium was not purely electromagnetic and must consist of particles. He named these particles neutrons because they had no charge and their mass was almost equal to protons.
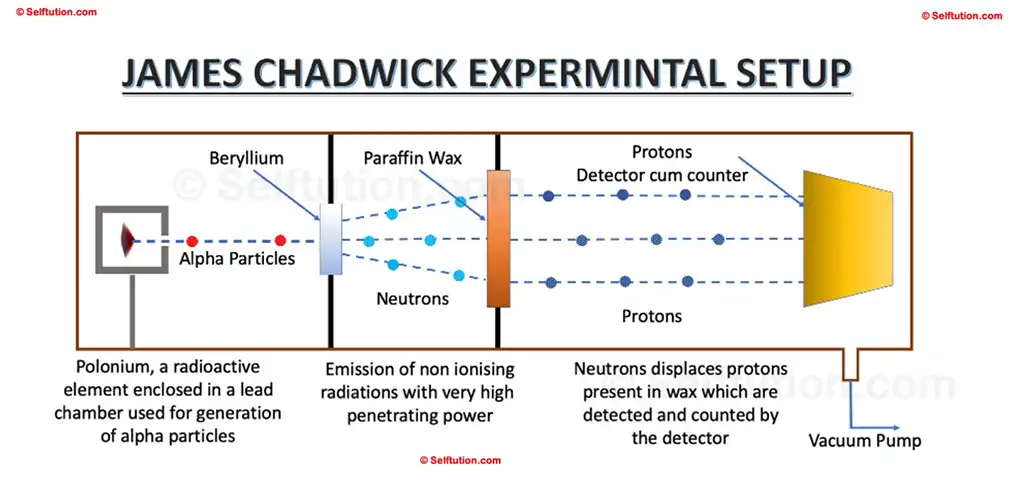
James Chadwick’s Experimental Setup for the Discovery of Photons and Neutrons
Chadwick’s discovery of neutrons provided answers to several unresolved questions in atomic theory and led to the presentation of a revised atomic model. The key features of his model include:
- The existence of a third type of subatomic particle, neutrons, within the nucleus. Neutrons have neither a positive nor a negative charge.
- Neutrons play a crucial role in maintaining the stability of the nucleus by mitigating the repulsive forces between positively charged protons.
- The greater mass of the nucleus is due to the presence of neutrons, which are approximately the same size as protons.
- Atoms of the same element can have different numbers of neutrons, leading to the formation of isotopes.
Chadwick’s work significantly advanced the understanding of atomic structure and addressed many of the lingering questions from previous atomic models.
5.3 Symbol & Properties of Neutron
A neutron is denoted as 0n1, where the superscript 1 represents 1 amu (atomic mass unit) mass and the subscript ‘0’ represent electrically a neutron is neutral, i.e. it has no charge. The mass of a neutron is slightly more than that of a proton, i.e. 1.676 x 10-24 g compared to 1.672 x 10-24 g.