Difference Between Specific Gravity and Density
Understanding the difference between specific gravity and density is essential, especially for students, engineers, and science enthusiasts.
If you’ve ever picked up a rock and wondered why it feels heavier than a similarly sized piece of wood, you’ve already stumbled upon the concept of density. But what about specific gravity? Many people use these terms interchangeably, but they are not the same.
Let’s break it down in a way that’s easy to grasp.
What is Density?
Let’s start with density, a concept we encounter daily. Imagine you have two identical-sized boxes: one filled with feathers and the other with bricks. Even though they take up the same amount of space, the box with bricks will feel much heavier. Why? Because bricks have a higher density than feathers.
Definition of Density
Density is defined as the mass of an object per unit volume. Mathematically, it is expressed as:
Density (ρ) = Mass (m) / Volume (V)
The standard unit of density in the SI system is kilograms per cubic meter (kg/m³), but you may also see it in grams per cubic centimeter (g/cm³) or pounds per cubic foot (lb/ft³), depending on the context.
Why Does Density Matter?
Density helps us understand why some objects float while others sink. For example:
- Wood floats on water because its density is lower than water’s.
- A steel nail sinks because steel is denser than water.
This principle is crucial in shipbuilding, aviation, and even cooking (think about oil floating on water in salad dressing!).
What is Specific Gravity?
Now that we understand density, let’s introduce specific gravity. It sounds complex, but it’s a simple concept: Specific gravity is a comparison of the density of a substance to the density of water.
Definition of Specific Gravity
Mathematically, it is written as:
Specific Gravity (SG) = Density of Substance / Density of Water
Since it’s a ratio of two similar units (density to density), specific gravity has no unit. It’s just a number.
Why Compare to Water?
Water is used as a reference because it has a convenient density of 1 g/cm³ (or 1000 kg/m³) at standard temperature and pressure. This makes calculations easy:
- If an object has a specific gravity greater than 1, it is denser than water and will sink.
- If the specific gravity is less than 1, the object is less dense and will float.
For example:
- The specific gravity of gold is about 19.3, meaning gold is 19.3 times denser than water.
- The specific gravity of ice is 0.92, which explains why ice floats on water.
How Temperature and Pressure Affect Specific Gravity and Density
Density
One important aspect of density is that it changes with temperature and pressure. When a substance is heated, its particles move further apart, increasing its volume and reducing its density. This is why hot air balloons rise—the air inside is less dense than the surrounding cooler air. On the other hand, under high pressure, gases become denser because their particles are forced closer together.
Specific Gravity
Since specific gravity depends on the density of water, and water’s density changes with temperature, specific gravity values can vary slightly under different conditions. For example, warm water is less dense than cold water, so a liquid measured at a higher temperature may have a slightly lower specific gravity than when measured at a lower temperature.
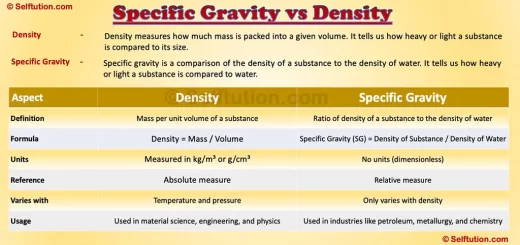
Key Difference Between Specific Gravity and Density
Now that we have clear definitions, let’s highlight the differences:
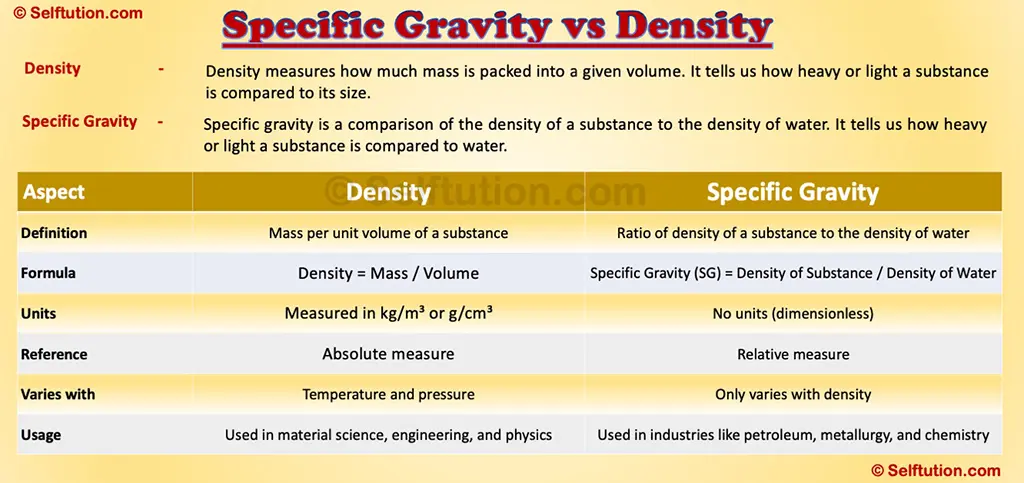
Specific Gravity vs Density
Why the Concept of Specific Gravity and Density is Important?
Understanding specific gravity and density isn’t just about passing science exams; it has real-world significance. Here’s why these concepts matter:
1. Everyday Decision Making
- When choosing construction materials, knowing their density helps in selecting strong yet lightweight materials.
- Checking the density of cooking oil or honey helps in food quality assessment.
- Knowing the density of different woods helps in selecting furniture or fuel-efficient firewood.
2. Industrial and Engineering Applications
- Engineers use density to design materials for airplanes, ships, and bridges.
- Fuel industries rely on specific gravity to determine fuel efficiency and quality.
- The density of metals determines their suitability for machinery and tools.
3. Environmental Impact of Specific Gravity and Density
- Oil spills occur because oil has a lower specific gravity than water, causing it to float and spread.
- Understanding how different materials interact with water helps in pollution control.
- Water treatment plants use density principles to separate impurities from water.
4. Medical and Health Applications
- Specific gravity is used in medical tests like urinalysis to assess hydration and kidney function.
- Bone density measurements help diagnose osteoporosis.
- The density of blood is used in diagnosing certain health conditions.
5. Role of Specific Gravity and Density in Space and Aviation
- Scientists study the density of planets to understand their composition.
- Fuel density in aviation ensures optimal aircraft performance.
- Understanding density variations in different atmospheres helps in space exploration.
6. Mining and Geology
- Geologists use specific gravity to identify minerals and rocks.
- Mining companies assess ore density to determine the economic feasibility of extraction.
- Petroleum engineers rely on density measurements to evaluate crude oil quality.
7. Food and Beverage Industry
- Brewers and winemakers use specific gravity to measure sugar content in fermentation.
- Dairy producers measure the density of milk to detect adulteration.
- The baking industry relies on density to achieve the right texture in bread and pastries.
Final Thoughts on Specific Gravity and Density
Although specific gravity and density are closely related, they serve different purposes. Density is an absolute measurement of mass per unit volume, while specific gravity is a relative measurement comparing a substance’s density to that of water.
Next time you see a floating log, a sinking rock, or honey dripping from a spoon, you’ll know exactly what’s happening. Science is everywhere—it’s just a matter of looking at it the right way!
You may also like…... Types of Chemical Reactions in Chemistry