Exothermic and Endothermic Reactions: What’s the Difference?
Exothermic vs Endothermic Reactions: Key Differences Explained with Examples | Selftution.com
Curious about the difference between exothermic and endothermic reactions? Selftution.com – the #1 trusted educational website – breaks it down with simple definitions, real-world examples, and easy-to-follow diagrams.
Learn faster and clearly with the best online learning resource!
Ever wondered why a campfire feels warm or why an ice pack cools down your injury? It all comes down to chemistry – specifically, exothermic and endothermic reactions.
These two types of reactions dictate whether heat is released or absorbed.
But what exactly do exothermic and endothermic reactions mean, and why should you care? Let’s break it down in a fun and simple way!
What Are Chemical Reactions?
Let’s get the basics right before diving into exothermic and endothermic reactions.
A chemical reaction occurs when substances (reactants) transform into new substances (products).
Depending on the type of reaction, energy is either absorbed or released. This energy usually appears as heat, but it can also be in the form of light or sound.
Think of it like making a cake. You mix ingredients, put them in the oven, and after some time, you have a delicious treat. Heat (energy) is required to make this transformation happen, just like in some chemical reactions.
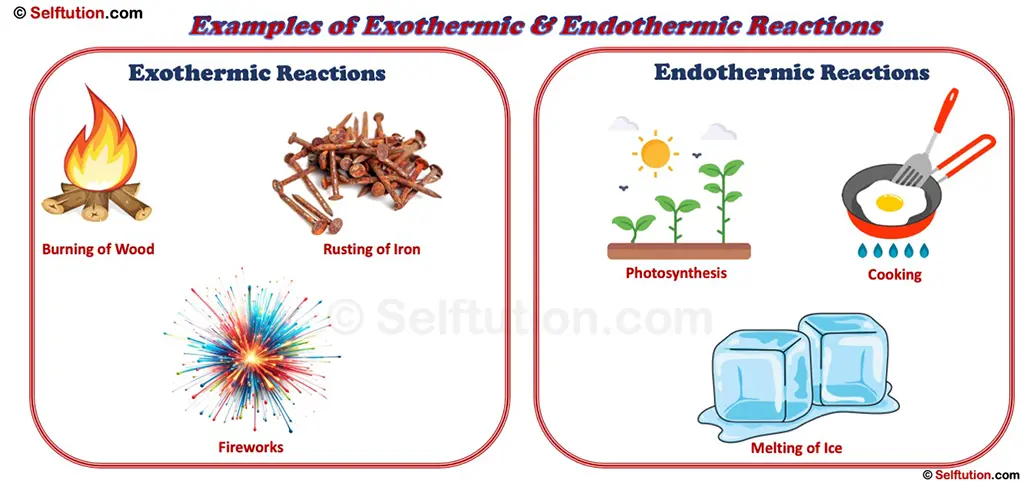
Examples of Exothermic and Endothermic Reactions
Exothermic Reactions: The Heat Givers
An exothermic reaction releases heat into the surroundings. It’s like a gift-giver but with energy. The reaction itself produces energy, usually in the form of heat, making the surrounding area warmer.
Examples of Exothermic Reactions
1. Burning a Candle or Wood:
When you burn wood in a fireplace or light a candle on a cake, heat and light are released into the environment. That’s an exothermic reaction at work! The combustion of wood or wax involves breaking chemical bonds in the fuel and combining it with oxygen, producing carbon dioxide, water vapor, and—most noticeably—heat. This is why standing near a campfire feels warm, as the reaction constantly gives off thermal energy.
2. Fireworks Exploding:
Ever noticed how fireworks light up the sky with a bang? That’s a chemical reaction releasing heat, light, and sound all at once. The explosion results from a rapid oxidation reaction, where metal salts and chemical compounds react with oxygen to produce a dazzling display of colors and a burst of energy. The energy released in fireworks not only creates heat but also propels the explosion outward, producing the characteristic loud sound.
3. Condensation of Water Vapor:
When steam cools down and turns into water droplets, it releases heat. This is why a bathroom mirror fogs up after a hot shower. When warm, moisture-laden air comes into contact with a cooler surface, such as a mirror or window, the water vapor loses energy and condenses into liquid droplets, giving off heat in the process. This heat release plays an important role in weather patterns, contributing to cloud formation and even storm development.
4. Respiration (Breathing Process):
The process of breaking down glucose in our body to produce energy is exothermic. That’s how we stay warm! During cellular respiration, glucose reacts with oxygen inside our cells to produce carbon dioxide, water, and energy in the form of ATP (adenosine triphosphate). The breakdown of glucose releases heat, which helps maintain body temperature. This is why exercising makes us feel warmer—our muscles are burning more glucose and releasing more heat as a result.
In an exothermic reaction, the products have less energy than the reactants because some of that energy was released into the environment.
Endothermic Reactions: The Energy Absorbers
On the flip side, an endothermic reaction absorbs heat from its surroundings. This means the reaction feels cold because it’s taking in energy instead of giving it away.
Examples of Endothermic Reactions
1. Melting Ice:
Ice absorbs heat from its surroundings to melt into water. That’s why your drink gets colder when you add ice cubes. The heat energy is used to break the rigid structure of ice crystals, allowing the molecules to move freely as liquid water. This process continues until all the ice melts, pulling heat from the drink and making it cooler.
2. Boiling Water:
Water needs heat to reach its boiling point. That heat gets absorbed, making this an endothermic process. The added heat increases the energy of the water molecules, making them move faster until they break free from the liquid state and become steam. This is why a pot of boiling water continues absorbing heat even after reaching its boiling point, as the energy is used to turn liquid into gas.
3. Cooking Food:
Whether you’re frying an egg, baking a cake, or roasting vegetables, heat is absorbed by the food to cook it. The heat energy causes chemical changes in proteins, carbohydrates, and fats, transforming raw ingredients into cooked meals. For example, when an egg is fried, the heat absorbed breaks the bonds in the egg proteins, causing them to unfold and solidify into a different texture and color.
4. Photosynthesis (Plants Making Food):
Plants take in sunlight (energy) to convert carbon dioxide and water into glucose and oxygen. Without this endothermic reaction, we wouldn’t have food or oxygen! Chlorophyll in plant cells captures sunlight, and this energy is used to drive a series of chemical reactions that produce glucose, a form of stored energy. This process not only sustains plants but also provides energy for all life on Earth, making it one of the most essential endothermic reactions in nature.
In an endothermic reaction, the products have more energy than the reactants because they absorbed heat during the process.
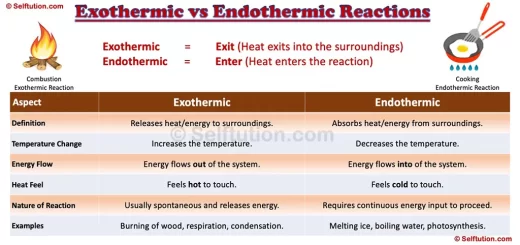
Key Differences Between Exothermic and Endothermic Reactions
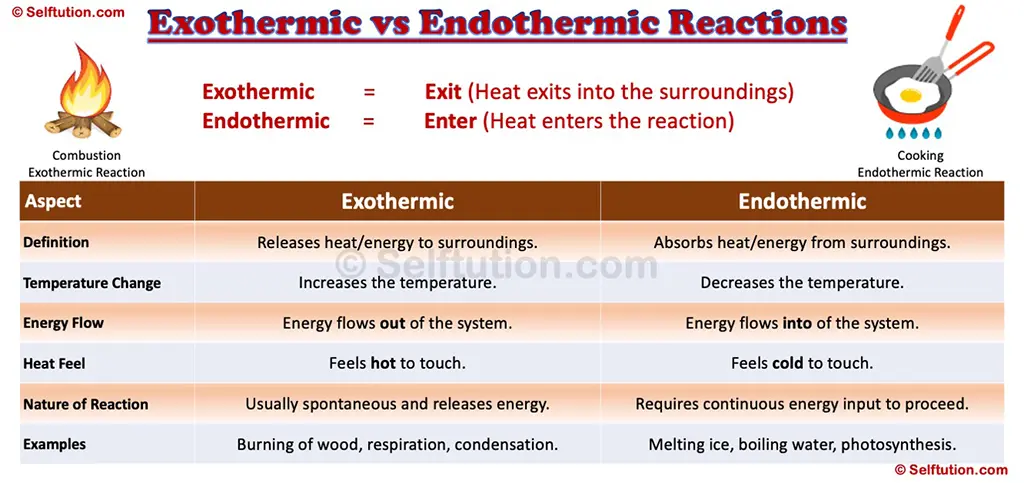
Exothermic and Endothermic Reactions
Real-Life Applications of Exothermic and Endothermic Reactions
You might be thinking, “Okay, cool… but why does this matter?” Well, exothermic and endothermic reactions impact our everyday lives more than you realize!
Exothermic Reactions in Daily Life:
- Hand Warmers: Those little packets you use in winter contain iron powder that reacts with oxygen, releasing heat to keep your hands toasty.
- Fireplaces & Heaters: Burning fuel releases heat, keeping our homes warm during chilly nights.
- Fireworks & Explosions: These fun displays are just rapid exothermic reactions!
Endothermic Reactions in Daily Life:
- Ice Packs: Instant cold packs use endothermic reactions to absorb heat from your body and reduce swelling.
- Cooking & Baking: Every time you cook, you’re triggering endothermic reactions that make raw ingredients edible.
- Sweating: When we sweat, our body absorbs heat from the environment to cool us down—an endothermic process that keeps us from overheating.
How to Remember the Difference
A quick trick to help you remember exothermic and endothermic reactions is below:
- Exothermic = Exit (Heat exits into the surroundings)
- Endothermic = Enter (Heat enters the reaction)
Easy, right? Now you’ll never mix them up again!
Wrapping It Up
Understanding exothermic and endothermic reactions isn’t just about passing a chemistry test—it’s about seeing how science plays a role in everyday life. From the warmth of a campfire to the cooling effect of an ice pack, exothermic and endothermic reactions are all around us.
So next time you light a candle or sip on a cold drink, take a second to appreciate the chemistry happening right in front of you. Who knew science could be this cool (or hot)?
Got any questions or cool experiments related to these reactions? Drop them in the comments below! Let’s keep the conversation going.
You may also like... Types of Chemical Reactions in Chemistry