Elements and Compounds | Simplified for kids
Elements and Compounds Explained for Kids – Fun, Simple & Engaging!
Discover the difference between elements and compounds with easy examples, colorful diagrams, and interactive quizzes – only on Selftution.com, the best educational website for clear, kid-friendly learning!
Welcome to Selftution.com – Where young minds explore science with joy!
So, let’s begin.
Kids often ask, What are elements and compounds? So here is the basic definition of an element and a compound that kids can easily remember.
Definition of Elements and Compounds
An element is a substance that has only one kind of atom, whereas a compound has two or more different kinds of atoms.
Topics Covered:
- Introduction
- What is an element?
- Definition of an element for kids
- Symbols of elements
- Molecules of elements
- What is a compound?
- Definition of a compound for kids
- Molecules of compounds
- Properties of elements and compounds
- Formulae of elements and compounds
- Difference between an element and a compound
INTRODUCTION
There are millions of substances in the world, each with unique characteristics. In chemistry, we explore the nature and properties of these substances to harness their potential for the benefit of humanity.
Given the vast number of substances, studying each one individually is impractical. Therefore, we categorize them into two main groups: elements and compounds. This classification helps streamline our study and understanding of their properties and behaviors.
Skip to >> The difference between element and compound for kids
WHAT IS AN ELEMENT?
Definition of an element for kids –
An element is a substance that cannot be broken into two or more simpler substances by any physical or chemical method. It consists of atoms of the same kind.
Elements are fundamental substances that form all the different materials around us. They are considered basic because they cannot be broken down into simpler substances physically or chemically. Each element consists of only one type of atom, meaning that no matter how much we divide an element, it will always result in the same kind of atoms. This indivisibility at the atomic level defines elements and distinguishes them from compounds, which are made up of two or more types of atoms chemically combined.
Let’s understand with an example-

House Construction Material
We need materials like mud, cement, bricks, sand, steel, and wood to build a house. By varying the quantities and ratios of these materials, we can construct anything from a simple house to a massive building. Similarly, in nature, elements combine in various ways to form all kinds of substances.
Examples of elements include hydrogen, helium, carbon, oxygen, calcium, nitrogen, sodium, iron, silver, gold, and mercury. To date, we have discovered a total of 118 elements. Out of these, 92 occur naturally in rocks, soil, air, and water, while scientists have created the remaining 26 artificially in laboratories.
Symbols of Elements
Scientists use symbols to represent various elements, instead of their names. For example, (H) stands for Hydrogen, (O) for Oxygen, (C) for Carbon, (N) for Nitrogen, and so on. We use symbols because when different elements combine to form compounds then it is easy to represent them by symbols.
Let’s understand it by an example,
To represent a molecule of sugar, we can write it either as ‘C12H22O11‘ or as ‘12 atoms of carbon, 22 atoms of hydrogen, and 11 atoms of oxygen ‘. The former method is better.
For a complete list of elements and their symbols, refer to the periodic table. The periodic table gives the complete list of 118 elements along with other important information.
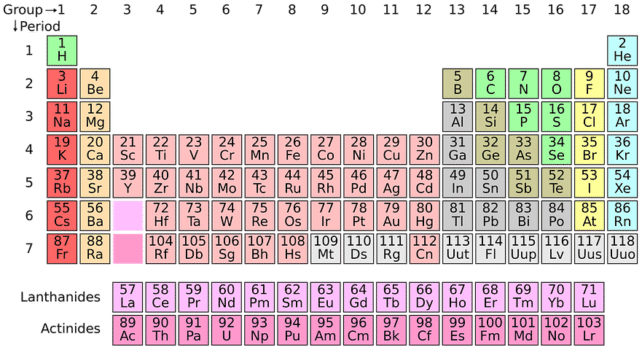
Periodic Table
Molecules of elements
A molecule of an element can be monoatomic, diatomic, or polyatomic, but it will always have atoms of the same kind. For example – Helium (He), Hydrogen (H2), Phosphorus (P4), Sulphur (S8), etc.
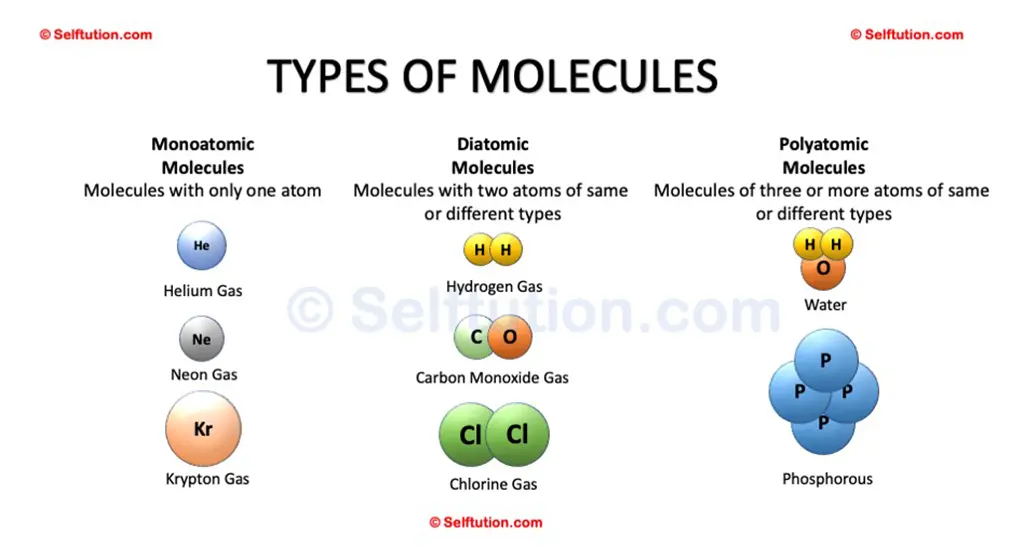
Examples of different types of molecules – Monoatomic, Diatomic, and Polyatomic
Skip to >> The difference between element and compound for kids
WHAT IS A COMPOUND?
Definition of a compound for kids-
When a molecule of a pure substance contains atoms of two or more elements combine in the fixed ratio, it is said to be a compound.
To understand a compound, first consider this fact. We know that the English alphabet has 26 letters. By combining these 26 letters, we can make millions of English words. In much the same way, you can combine 118 different kinds of elements to make an endless number of compounds. Therefore, a compound is a substance formed when two or more elements combine in a fixed ratio. It is a new substance formed from its elements. For example, two atoms of the element hydrogen (H) and one atom of the element oxygen (O) combine to form a molecule of water (H2O), which is a compound.
We can break a molecule of a compound to get pure elements. However, this breakup is not possible with simple physical methods. In compounds, chemical bonds join atoms together. These bonds are very strong and difficult to break. Thus, to get original elements (or atoms) from compounds, we need to apply chemical methods. For example, to break the molecule of water into its elements, hydrogen and oxygen, we need to pass an electric current through it.
Molecules of Compounds
The molecule of the compound always contains two or more atoms of different kinds. For example,
- Hydrogen (H) and oxygen (O) make water (H2O),
- Carbon (C) and oxygen (O) make carbon dioxide (CO2),
- Hydrogen(H) and chlorine (Cl) make hydrochloric acid (HCl).
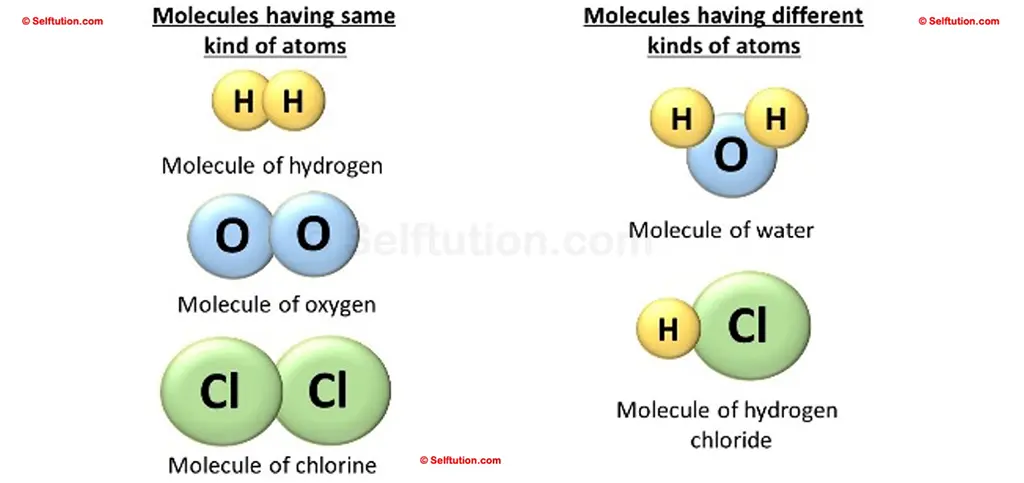
Molecules of elements and compounds
PROPERTIES OF ELEMENTS AND COMPOUNDS
Compounds exhibit properties markedly different from the properties of the elements that constitute them. For instance, a molecule of water (H₂O) is formed by combining two hydrogen (H) atoms with one oxygen (O) atom. While hydrogen and oxygen are gases under normal conditions, water is a liquid. This demonstrates how the elements, by combining in fixed proportions, can create a substance with entirely new and distinct physical and chemical properties.
Additionally, the behavior of compounds can be drastically different from their constituent elements. Hydrogen and oxygen, when mixed, can create a highly flammable and explosive environment if ignited. Conversely, water, composed of hydrogen and oxygen, is commonly used to extinguish fires. This stark contrast illustrates how chemical bonding and molecular structure profoundly influence the characteristics and uses of compounds, highlighting the fascinating complexity of chemical interactions in nature.
FORMULAE OF ELEMENTS AND COMPOUNDS
Formulas are a short way of representing molecules of elements and compounds. They help in understanding the structure of molecules. Formulas are more relevant for compounds than elements, as they consist of atoms of different elements. The formula for compound gives the following information:
- It tells which elements are present in a compound, and
- It tells the number of atoms of each element present in a compound.
For example, the formula ‘H2O’ represents water. It tells us that two atoms of hydrogen and one atom of oxygen make a molecule of water.
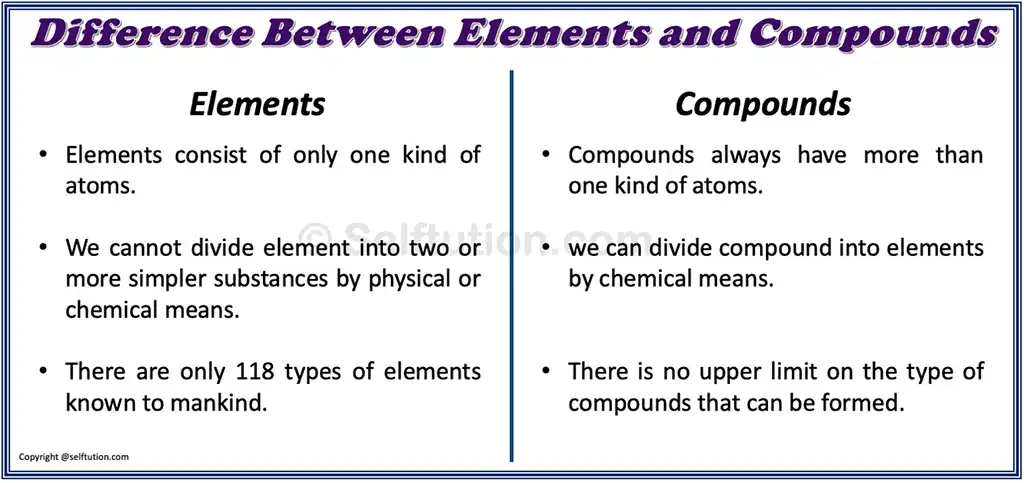
DIFFERENCE BETWEEN ELEMENTS AND COMPOUNDS FOR KIDS
- Elements consist of only one kind of atom, whereas compounds always have more than one kind of atom.
- We cannot divide elements into two or simpler substances by physical or chemical means, whereas we can divide compounds into elements by chemical means.
- We use a symbol to represent an element, whereas a chemical formula represents a compound.
- There are only 118 types of elements known to mankind, whereas there is no upper limit on the types of compounds that can be formed.
Difference between elements and compounds in tabular form: