Heat and Temperature: What’s the Difference?
In everyday life, we frequently use the words “heat” and “temperature” to describe how hot or cold something feels. But have you ever wondered what the real difference is between the two? They may sound similar, and we often use them interchangeably, but they are very different scientifically.
In this blog, we’ll dive into the concepts of heat and temperature, break them down, and explain their differences in simple, easy-to-understand terms.
What is Heat?
Let’s start with heat. Heat is a form of energy that can transfer from one object to another due to a difference in their temperature. For example, when you touch a hot cup of tea, the heat from the cup transfers to your hand, making it feel warm. Heat is quantified using units like joules or calories
Heat always flows from a warmer object to a cooler one. For instance, if you put an ice cube into a glass of warm water, the heat from the water will flow into the ice cube, causing it to melt. This is because the warm water has more heat energy, and it transfers that energy to the cooler ice cube.
If we observe at the microscopic level, heat is essentially the energy of motion of particles. All matter is made up of tiny particles like atoms and molecules. These particles are always moving, and the faster they move, the more heat energy they have. So, when you heat an object, you increase the speed of the particles inside it.
Types of Heat Transfer
Heat transfer takes place in three ways:
- Conduction
In conduction, heat transfer occurs due to direct contact between objects. For example, when you touch a hot metal pan, heat moves from the pan to your hand through conduction. The particles in the pan are moving rapidly, and when they come in contact with your skin, they pass on some of their energy, making your hand feel hot. - Convection
Convection happens when heat is transferred through fluids, like air or water. For example, when you boil water, the heat from the stove warms the water at the bottom of the pot. The warmer water then rises, and the cooler water moves down to take its place. This cycle of rising and falling water creates convection currents, distributing the heat throughout the water. - Radiation
Radiation transfers heat through electromagnetic waves, such as those from the sun. Unlike conduction and convection, radiation doesn’t require particles to transfer heat. That’s why you can feel the warmth of the sun on your skin even though there’s a vacuum of space between the Earth and the sun.

Which instrument is to be used for the measurement of temperature?
What is Temperature?
Now, let’s talk about temperature. Temperature is a physical quantity that measures how hot or cold the object is. It tells us how much energy the particles in an object have. When the particles are moving very quickly, the temperature is high. When the particles are moving more slowly, the temperature is lower.
Temperature is measured using thermometers, and there are several units of measurement for temperature, including Celsius (°C), Fahrenheit (°F), and Kelvin (K). In most of the world, Celsius is the most commonly used scale, while Fahrenheit is more common in the United States. The Kelvin scale is often used in scientific research.
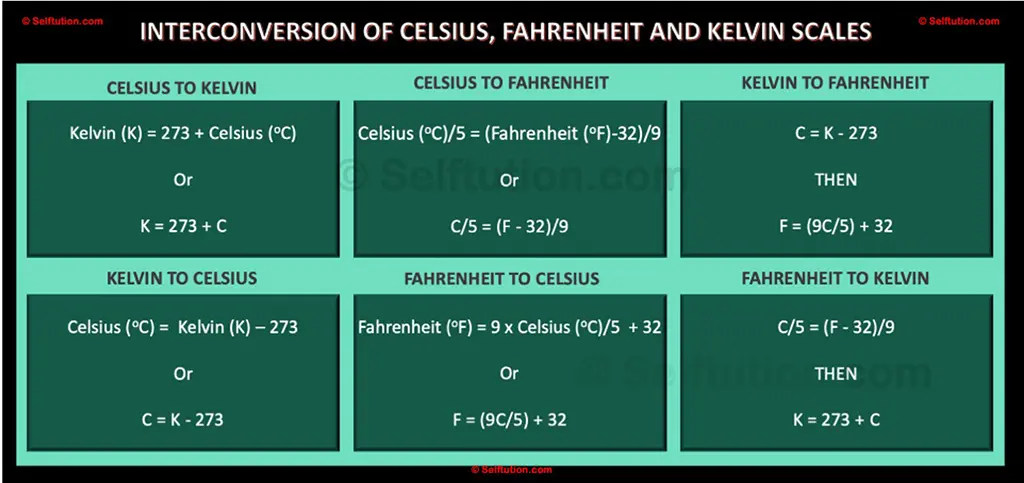
Temperature Scales, Celsius to Fahrenheit to Kelvin
Unlike heat, temperature is not a form of energy. Instead, it’s a way of measuring the average kinetic energy of the particles in an object. In simple terms, it tells us how fast the particles in the object are moving. If the particles are moving quickly, the object has a high temperature. If the particles are moving slowly, the object has a low temperature.
How Do We Measure Temperature?
We can measure temperature using thermometers. There are different types of thermometers, but the most common ones use liquids like mercury or alcohol. As the temperature rises, the liquid in the thermometer expands and moves up the scale, showing a higher temperature. When the temperature drops, the liquid contracts and moves down the scale.
Other thermometers, like digital ones, use electronic sensors to measure temperature more precisely. These thermometers are often used in modern-day devices like air conditioners, refrigerators, and medical equipment.

The image depicts various parts of the clinical thermometer – the stem, capillary tube, glass bulb mercury, and kink which prevents backflow of mercury
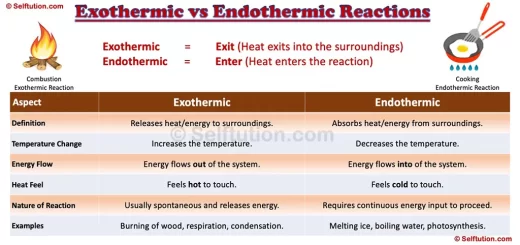
The Key Differences Between Heat and Temperature
Now that we know what heat and temperature are, let’s take a closer look at their key differences:
- Nature
Heat is a form of energy, whereas temperature is a measure of how much energy the particles in a substance have. Heat is something that flows, while temperature is something that tells us how hot or cold an object is. - Unit of Measurement
Heat is measured in joules (J) or calories, while temperature is measured in degrees Celsius (°C), Fahrenheit (°F), or Kelvin (K). - Direction of Flow
Heat always flows from a hotter object to a cooler one. Temperature, on the other hand, does not “flow.” It simply tells us how hot or cold something is. - Dependent vs. Independent
Heat depends on both the temperature of an object and its mass. For example, a large pot of boiling water contains more heat than a small cup of boiling water, even though they have the same temperature. Temperature, however, does not depend on the size or amount of the substance. It’s just a measure of the average energy of the particles. - Effect on Matter
Heat can cause a change in the state of matter. For example, adding heat to ice turns it into water, and adding even more heat turns water into steam. Temperature, however, is just a measure of how hot or cold a substance is and does not cause the substance to change its state.
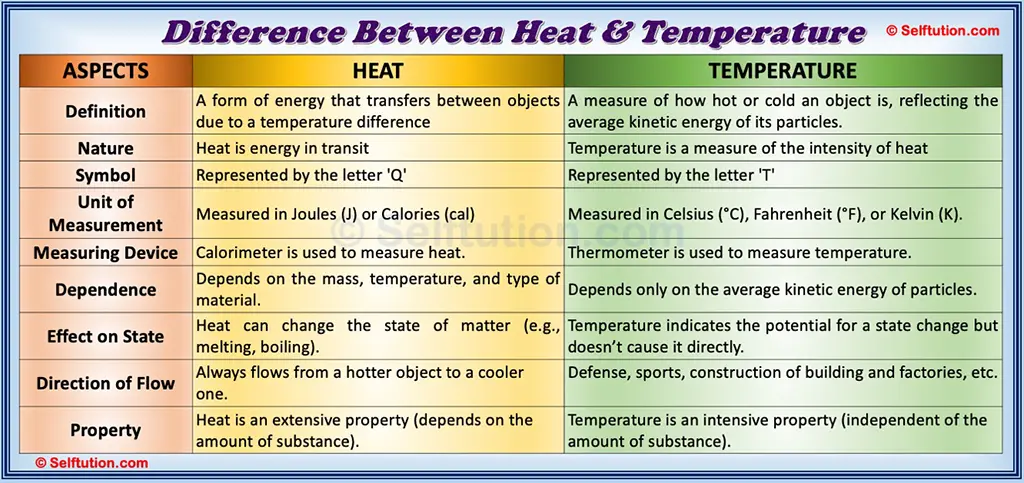
Heat vs Temperature
An Example: Boiling Water
Let’s consider the example of boiling water to clarify the difference between heat and temperature. When you heat a pot of water on the stove, you’re adding heat energy to the water. As the heat increases, the particles in the water start moving faster and faster. Once the water reaches 100°C (at sea level), it starts to boil.
At this point, even though you’re still adding heat, the temperature of the water remains the same (100°C) because the extra heat is being used to turn the water into steam. This is why temperature and heat are different. The temperature stays constant during the boiling process, but the heat continues to increase as the water changes state from liquid to gas.
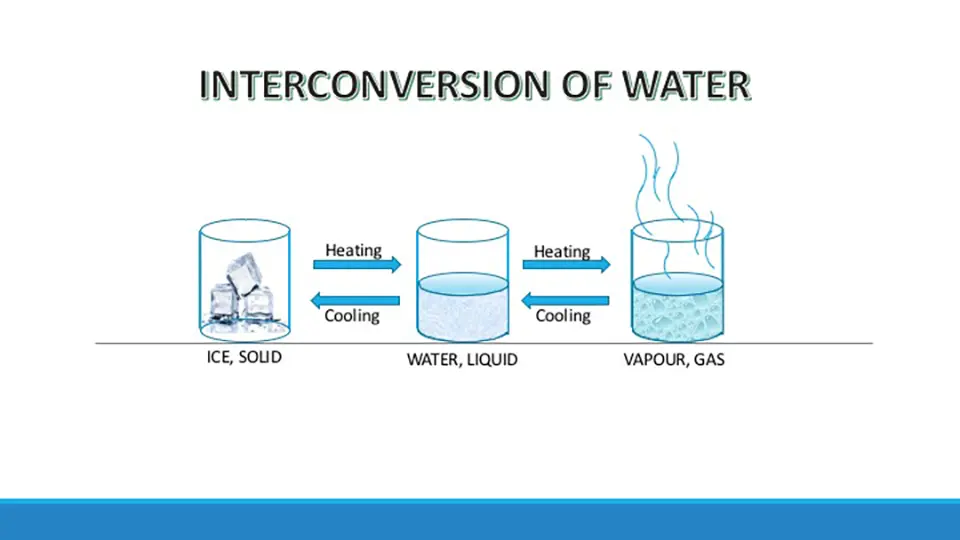
The picture depicts the interconversion of states of matter with an example of water. Here water, a form of matter undergoes interconversion from solid to liquid state and from liquid to gaseous state on heating. Similarly, on cooling vapor changes to a liquid and on further cooling to solid.
Understanding Heat & Temperature w.r.t. Specific Heat Capacity
Another important concept to understand when talking about heat and temperature is specific heat capacity. Specific heat capacity is the amount of heat energy required to raise the temperature of 1 gram of a substance by 1°C.
Different materials have different specific heat capacities. For example, water has a high specific heat capacity, which means it takes a lot of heat to raise its temperature. That’s why the ocean doesn’t warm up or cool down quickly. On the other hand, metals like iron have a low specific heat capacity, which means they heat up quickly with less energy.
This concept helps us understand why different materials respond differently to the same amount of heat. For example, if you place a metal spoon and a plastic spoon in a pot of boiling water, the metal spoon will get hot much faster than the plastic spoon, even though they are both in the same pot of water.
Heat and Temperature in Everyday Life
Understanding the difference between heat and temperature can help us make sense of the world around us. For example, when you’re cooking, you use heat to raise the temperature of food until it’s cooked. Similarly, when you step outside on a hot summer day, the sun’s rays are transferring heat energy to your skin, raising its temperature and making you feel warm.
In winter, we wear warm clothes to prevent the heat from our bodies from escaping into the cold air. This helps us maintain a comfortable body temperature.
In homes and buildings, we use heaters and air conditioners to control temperature. Heaters add heat to the air, raising the temperature, while air conditioners remove heat, lowering the temperature. Understanding these processes helps us use energy more efficiently and stay comfortable.
Conclusion
In summary, heat and temperature are closely related but very different concepts. Heat is a form of energy that can transfer from one object to another, while temperature is a measure of how hot or cold something is. Heat depends on the mass and temperature of an object, while temperature only depends on the average kinetic energy of the particles.
By understanding the difference between heat and temperature, we can better understand the natural processes that occur around us every day, from boiling water to weather changes. It’s a fundamental concept in science that helps explain many of the phenomena we observe in our daily lives.
For more such information, please visit our YouTube channel SELFTUTION