Difference in Physical and Chemical Change with Examples
In chemistry, all types of changes can broadly be classified into physical and chemical changes. In this post, we will learn about the difference in physical and chemical change with examples-
- The characteristics of physical and chemical changes with examples,
- The differences between physical and chemical changes, and
- How to know that chemical change has taken place?
- The burning of a candle is a physical or a chemical change?
PHYSICAL CHANGE
Physical change definition:
A physical change is a change in which no new substance is formed. The substance may change in shape, size, and physical state and appearance, but not in its chemical composition.
These changes may be temporary (reversible) or permanent (irreversible).
Skip to the chemical change definition
Skip the difference between physical and chemical changes
CHARACTERISTICS OF PHYSICAL CHANGE
- No new substance is formed.
- There is no change in the composition of the substance.
- The properties of the substance remain the same.
- The change is temporary or reversible but not always.
- There may be changes in size, shape, and state of substance during physical change.
- There may or may not be any exchange of energy during a physical change.
EXAMPLE OF PHYSICAL CHANGES
Some day-to-day examples of physical changes are – the drying of clothes, the ringing of a bell, the melting of ice cream, the melting of butter, the glowing of a bulb, the heating of electric iron, the beating of metals into sheets, the formation of clouds, cutting of the grass, boiling or freezing of water, sublimation of camphor, making of sugar solution, etc.

Examples of physical change.
Let us understand some of them in detail.
Example – 1: Interconversion of states of matter (Reversible physical change)
The interconversion of states of matter is a physical change. You might have observed that ice, when taken out of a refrigerator melts and changes into water, and when put back in the refrigerator, it freezes into ice again. During the melting or freezing of water, no new substance is formed, only the state of water changes.
Similarly, on boiling, water changes into steam. On cooling, the steam again changes back into the water. So again, there is only a change in the state of the water and no change in the chemical composition of water occurs.
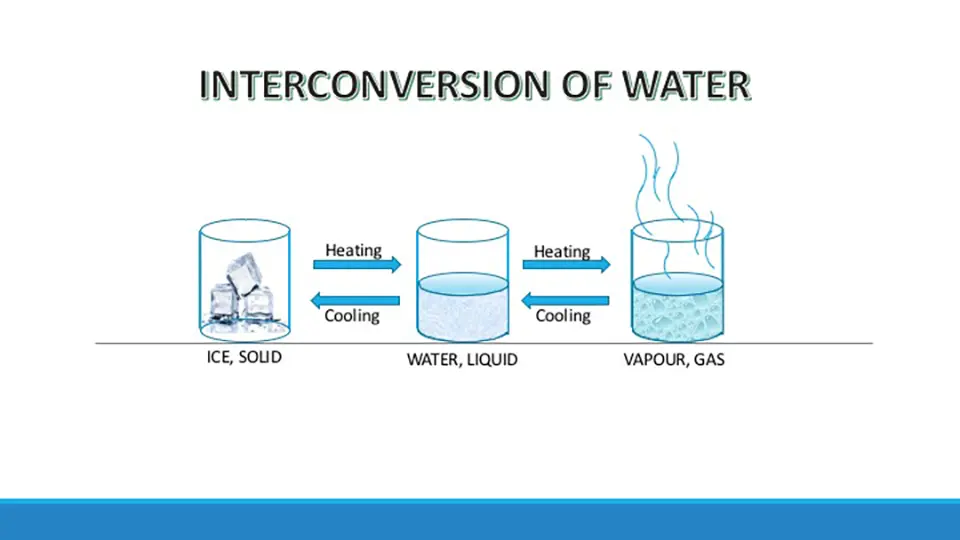
The picture depicts the interconversion of states of matter with an example of water. Here water, a form of matter undergoes interconversion from solid to liquid state and from liquid to gaseous state on heating. Similarly, on cooling vapor changes to a liquid and on further cooling to solid.
On removing the cause of change the substance returns to its original state. Thus, physical change is a reversible or temporary change.
Example-2: Crushing and making of chalk (Reversible physical change)
To observe a physical change with the help of chalk, crush some pieces of it into dust. Add a little water to the dust and make a thick paste of it. Roll this paste into the shape of a chalk piece, and let it dry. On drying, you will get back the chalk with its original properties. Therefore, physical change is a reversible change.
Example-3: Sublimation of Ammonium Chloride (Reversible physical change)
Sublimation is the process of changing a solid directly into gas on heating and vice-versa without passing through a liquid state. To demonstrate the same, take some ammonium chloride in a test tube and heat it gently. You will observe that solid changes into vapors. When the vapor touches the upper cooler part of the test tube it changes back to solid ammonium chloride. This shows that no new substance is formed. Thus, the sublimation of ammonium chloride is a physical reversible change.
However, physical change may not always be reversible. Some examples, of irreversible physical change, are –
Example – 4: Cutting of carrot into pieces (Irreversible physical change)
Take a carrot and cut it into pieces. What do you observe? The shape and size of the carrot have changed. Now observe the color of the carrot pieces and taste one of them. There will be no difference in the taste of the whole carrot and its pieces.
Thus, physical change only affects the physical form of a substance and the chemical composition of the final product remains identical to the original substance. However, in the present case, once the carrot is cut into pieces, we cannot obtain the original carrot. Thus, physical change may not be always reversible.
Example – 5: Cutting of paper into pieces (Irreversible physical change)
Take a piece of paper and cut it into four square pieces. Now cut each square piece further into four square pieces. Lay them on a table so that pieces acquire the shape of the original paper. Although the chemical composition of each piece of paper is similar to that of the original paper, even then you cannot join all the pieces back to make the original paper without cut marks. Thus, cutting paper is an irreversible physical change.
Back to the physical change definition
Skip to the difference between physical and chemical changes
CHEMICAL CHANGE
Chemical change definition:
A chemical change is a permanent change in which new substance(s) are formed. The new substance(s) shows composition and properties which are completly different from those of the original substance.
CHARACTERISTICS OF CHEMICAL CHANGE
- The change is permanent and irreversible.
- One or more new substances are formed.
- There is an exchange of energy during a chemical change. This means the heat or light or both might be given out or consumed.
EXAMPLES OF CHEMICAL CHANGES
Some day-to-day examples of chemical changes are – the rusting of iron, the ripening of fruits, the bursting of crackers, the souring of milk, the burning of paper or coal, the decomposition of organic matter, etc.

Examples of chemical change.
Let us understand some of them in detail.
Example-1: Burning of paper and coal (Irreversible chemical change)
Take a piece of paper and burn it. It turns into ash. This is a new substance, whose properties are different from the paper. The ash cannot change into paper again. Therefore, the burning of paper is a chemical change.
Coal is primarily made of carbon. On burning, coal reacts with oxygen present in the air to form carbon dioxide gas. We cannot change carbon dioxide back to carbon by any physical method. Therefore, the burning of coal is a chemical change.
Example-2: Rusting of iron (Irreversible chemical change)
Take an iron nail and bring it near a magnet. The magnet attracts the nail. Now leave the nail in tap water for a few days. You will notice that the nail develops a reddish-brown coating all over it. This coating is called rust. Rust is a hydrated oxide of iron, a new substance. It forms due to a reaction of iron with oxygen dissolved in water.
Again bring the nail near the magnet. But now magnet does not attract nails toward itself. This shows that rust is a new substance that is non-magnetic. You cannot get iron back from rust with any physical method. Thus, rusting of iron is an irreversible and permanent chemical change.
Example – 3: Heating of sugar (Irreversible chemical change)
Put some sugar in a pan and heat it. You will notice that sugar first melts and then changes into a reddish-brown solid. On prolonged heating, it turns black and gets charred. Now stop heating and taste it on cooling. You will notice it is not sweet anymore. On heating, the sugar converts to black residue called charcoal, and some gaseous products like carbon dioxide and water. Charcoal is a form of carbon, an element. You cannot get sugar back from charcoal with any physical method. Therefore, the heating of sugar is an irreversible and permanent chemical change.
HOW TO KNOW THAT CHEMICAL CHANGE HAS TAKEN PLACE?
A chemical change is usually accompanied by a change in color, the evolution of gas, and the release or absorption of energy.
Change in color: Ripening of fruits is a chemical change. The color of raw fruits changes during ripening. Heating of sugar and burning of paper gives a black substance.
Evolution of a gas: On the reaction of metals like zinc with dilute hydrochloric acid, the evolution of hydrogen gas takes place. Heating of sugar results in the evolution of carbon dioxide gas.
Release or absorption of energy: During a chemical change, energy is evolved or absorbed in the form of heat, light, or sound. An explosion of a cracker is a chemical change, that produces heat, light, and sound energy.
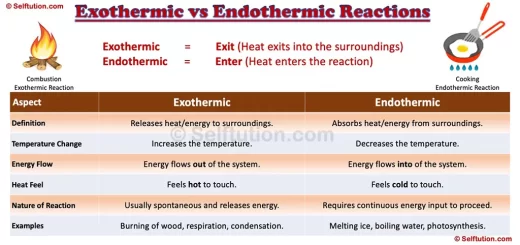
DIFFERENCE BETWEEN PHYSICAL AND CHEMICAL CHANGE
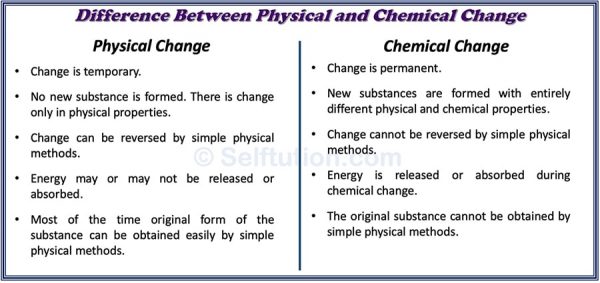
Difference in physical and chemical changes
BURNING OF CANDLE IS A PHYSICAL OR A CHEMICAL CHANGE?
The burning of a candle is an example of simultaneous physical and chemical change.
When a candle is lit, the wax melts and turns into a liquid state. As the molten wax drops on the floor or table, it solidifies again. Therefore, this is a physical change.
Simultaneously, some of the molten wax rises up the wick, turns into vapor, and burns with the flame to form two new substances carbon dioxide and water vapor. The amount of wax that gets burned escapes in the form of gases and the candle becomes smaller and smaller. This is a chemical change.
Thus, the melting of wax is a physical change and the burning of wax is a chemical change.
Back to the physical change definition
Back to the chemical change definition
For more such information, please visit are YouTube channel SELFTUTION